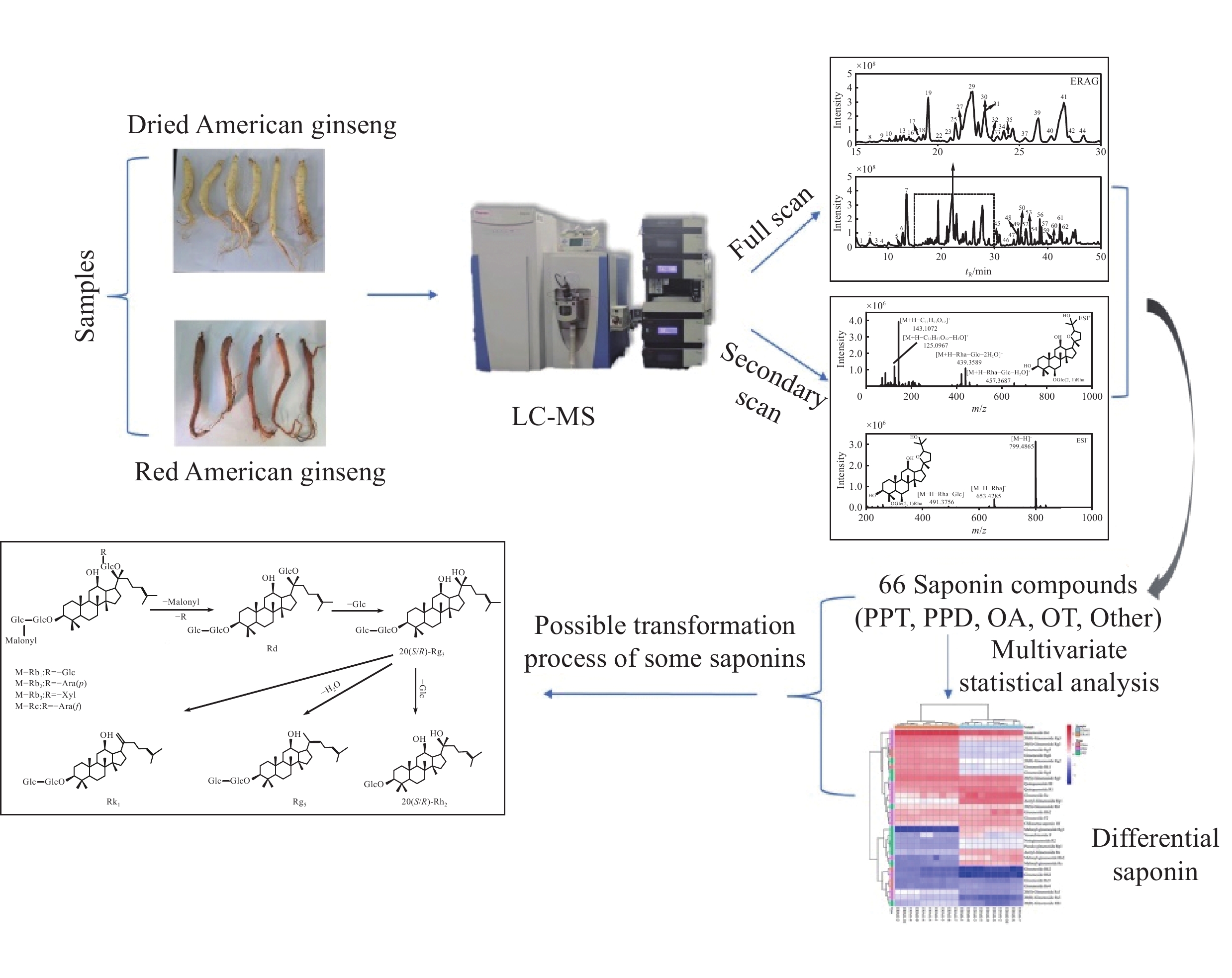
-
西洋参为五加科植物西洋参Panax quinquefolium L.的干燥根,性甘、微苦、凉,归心、肺、肾经,有补气养阴、清热生津之功效[1-2]。现代研究表明,西洋参具有多种生物活性,如抗氧化、抗糖尿病、抗炎、抗癌等[3-7]。《本草纲目拾遗》记载:“洋参似辽参之白皮炮丁,味类人参,惟性寒,宜糯米饭上蒸用,甘苦,补阴退热,姜制,益元扶正气。”人参皂苷作为西洋参的主要活性成分[8-10],是其发挥独特药理作用的物质基础。目前,常见的西洋参炮制品有生晒西洋参、红西洋参等,其中,鲜西洋参蒸制干燥1次制得红西洋参[11]。已有研究表明,在红西洋参的加工过程中,由于温度影响,会发生皂苷间的生物转化[12],主要为原型皂苷到稀有皂苷的转化、脱乙酰基和去丙二酰基等化学反应[13]。
本研究基于超高效液相色谱-四极杆-静电场轨道阱高分辨质谱(UHPLC-Q-Orbitrap-MS/MS)技术,通过高分辨质谱数据及二级质谱,并结合文献[14-18]分析,鉴定生晒西洋参与红西洋参中的皂苷类成分,总结不同类型皂苷的质谱裂解规律,结合主成分分析和正交偏最小二乘法判别分析法分析2种西洋参炮制品中的差异性皂苷,以期为西洋参资源的开发和合理利用提供理论依据。
-
Ultimate 3000超高效液相色谱仪、Thermo Q-Exactive Orbitrap质谱仪:美国Thermo Fisher公司产品;电子分析天平:赛多利斯科学仪器(北京)有限公司产品;KM-5200DE型超声清洗仪:昆山市超声仪器有限公司产品。
人参皂苷Re、Rd、Rb1、Rg1、Rg5、Rk1、20(R)-Rg2、20(S)-Rg3、20(R)-Rg3、20(S)-Rh1、20(R)-Rh1、20(S)-Rh2及拟人参皂苷F11对照品(批号B21055、B21054、B21050、B21057、B21044、B21065、B21727、B21059、B21759、B21061、B21728、B21062、B20902,纯度均大于98%):上海源叶生物科技有限公司产品;乙腈、甲醇(色谱纯):美国Thermo Fisher公司产品;甲酸:美国Sigma公司产品;水为超纯水。
-
生晒西洋参的制备:取鲜参,在50 ℃烘箱中干燥。
红西洋参的制备:取鲜参,置于100 ℃人参蒸柜中蒸制3 h,取出后置于50 ℃烘箱中烘干。
-
精密称取适量的人参皂苷Re、Rd、Rb1、Rg1、Rg5、Rk1、20(R)-Rg2、20(S)-Rg3、20(R)-Rg3、20(S)-Rh1、20(R)-Rh1、20(S)-Rh2及拟人参皂苷F11对照品,加入70%甲醇,定容至5 mL容量瓶中,超声溶解,即得对照品溶液。
取0.1 g西洋参粉末(过3号筛),精密称定,置于具塞锥形瓶中,精密加入5 mL 70%甲醇,密封,称定质量;超声提取45 min,放冷,再次称定质量;用70%甲醇补足失重,摇匀,过0.22 μm微孔滤膜,取续滤液,即得供试品溶液。
-
Supelco C18色谱柱(3.0 mm×50 mm,2.7 μm);流动相:0.1%甲酸水溶液(A)-乙腈(B);梯度洗脱程序:0~12 min(82%~80%A),12~14 min(80%~70%A),14~24 min(70%~68%A),24~29 min(68%~67%A),29~49 min(67%~25%A),49~55 min(25%~0%A),55~55.01 min(0%~82%A),55.01~60 min(82%A);柱温35 ℃,进样量5 μL;流速0.3 mL/min。
-
电喷雾离子源(ESI),正、负离子模式,鞘气流速10.5 L/min,辅助气流速3 L/min,离子导入射频电平(S-lens RF level)为55,毛细管电压±3.5 kV,毛细管温度350 ℃。全扫描参数设置:质量扫描范围m/z 150~2 000,分辨率70 000,自动增益控制(AGC)3×106,最大进样时间(IT)100 ms。二级质谱扫描参数设置:分辨率17 500, AGC 1×105, IT 50 ms,环数为5,分离窗口m/z 4.0,归一化碰撞能(NCE)25~55。
-
采用Analysis Base File Converter 软件对UHPLC-Q-Orbitrap MS/MS原始数据进行文件格式转换,通过MS-DIAL软件对数据进行峰提取、识别、对齐和归一化等预处理,得到包含质荷比、保留时间和峰面积的数据集。使用MetaboAnalyst-6.0进行主成分分析(PCA),使用SIMCA-14.0进行正交偏最小二乘法判别分析(OPLS-DA),根据投影变量重要性(VIP)大于1和student-t检验结果P值小于0.05筛选差异皂苷,并通过GraphPad Prism 9软件对比生晒西洋参和红西洋参中差异皂苷的相对含量。
-
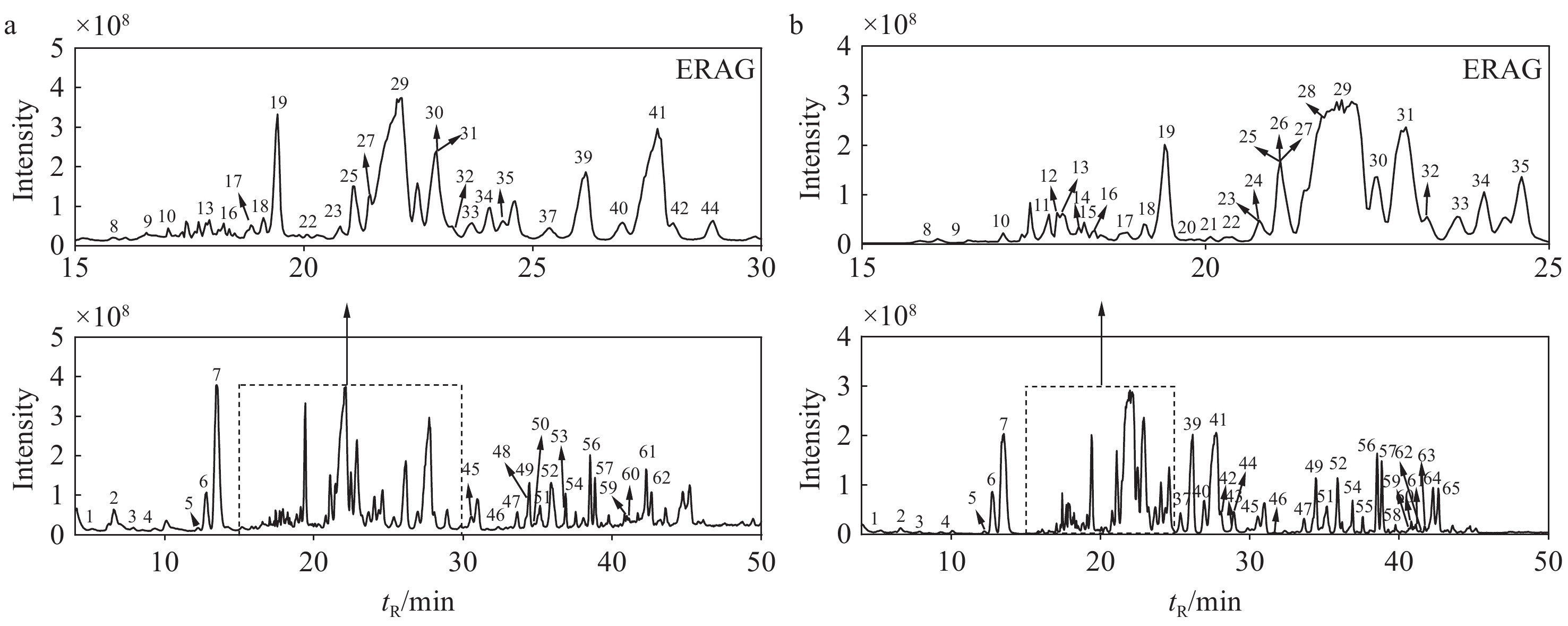
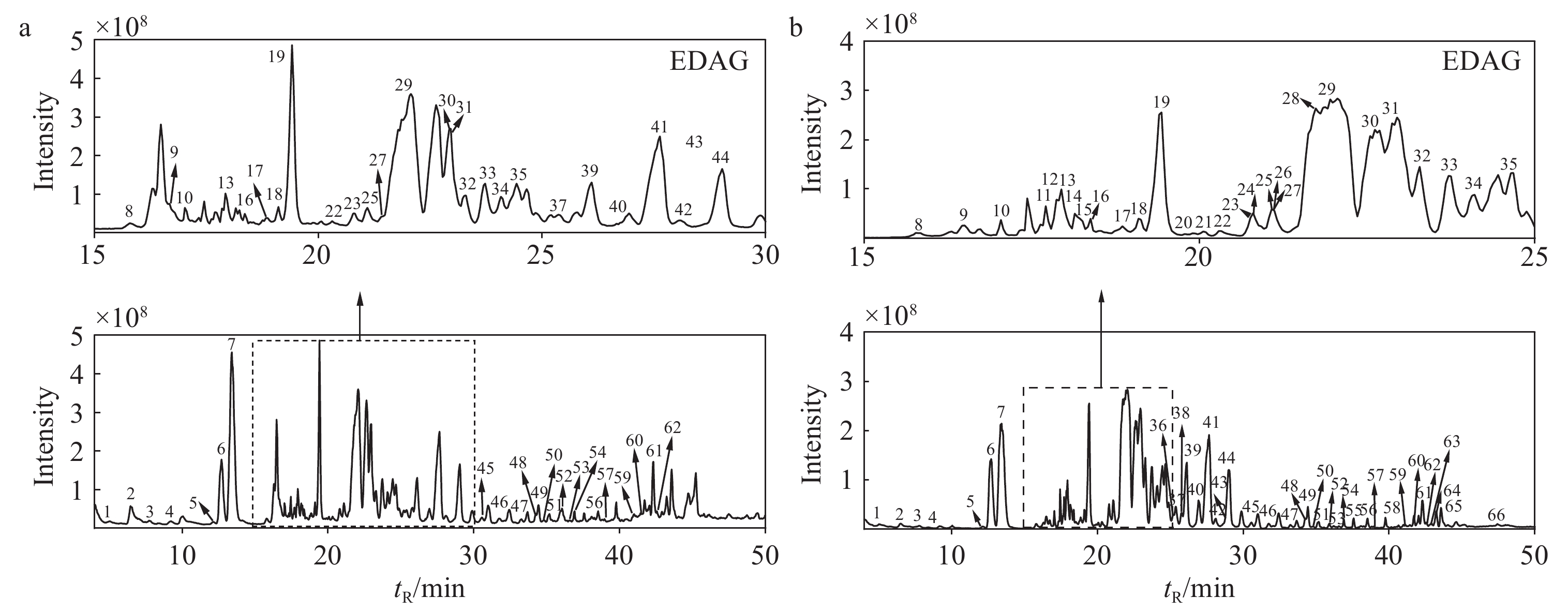
在正、负离子模式下,共鉴定出生晒西洋参和红西洋参中66种皂苷类成分,包括33种原人参二醇型(PPD)、18种原人参三醇型(PPT)、4种齐墩果烷型(OA)、4种奥克梯隆型(OT)和7种其他类型人参皂苷。提取的生晒西洋参和红西洋参的总离子流图分别示于图1、2,每种皂苷的详细信息列于表1。
-
按四环母核上C-3、C-6、C-12、C-20 4个羟基中有无C6-OH,可将人参皂苷分为PPD型和PPT型,其苷元上常连有1~6个糖基。PPD型人参皂苷的糖基一般以β-OH的形式连接在C-3、C-20位;PPT型人参皂苷的糖基通常以α-OH的形式连接在C-6位或以β-OH的形式连接在C-20位。在负离子模式下,人参皂苷一般以准分子离子[M−H]−和[M+HCOO]−的形式存在。对于PPD型人参皂苷,[M−H]−离子竞争丢失C-20、C-3位上的糖基;对于PPT型人参皂苷,[M−H]−离子竞争丢失C-20、C-6位上的糖基。人参皂苷中具有不同连接形式的己糖(葡萄糖,glucose,Glc)、脱氧己糖(鼠李糖,rhamnose,Rha)和果糖(阿拉伯糖,arabinose,Ara;木糖,xylose,Xyl),当皂苷的糖苷键断裂时,所丢失的糖基类型可以通过m/z 162(−Glc)、146(−Rha)、132(−Ara或−Xyl)的损失来确定。正离子模式下,人参皂苷一般以准分子离子[M+Na]+和[M+H]+的形式存在。
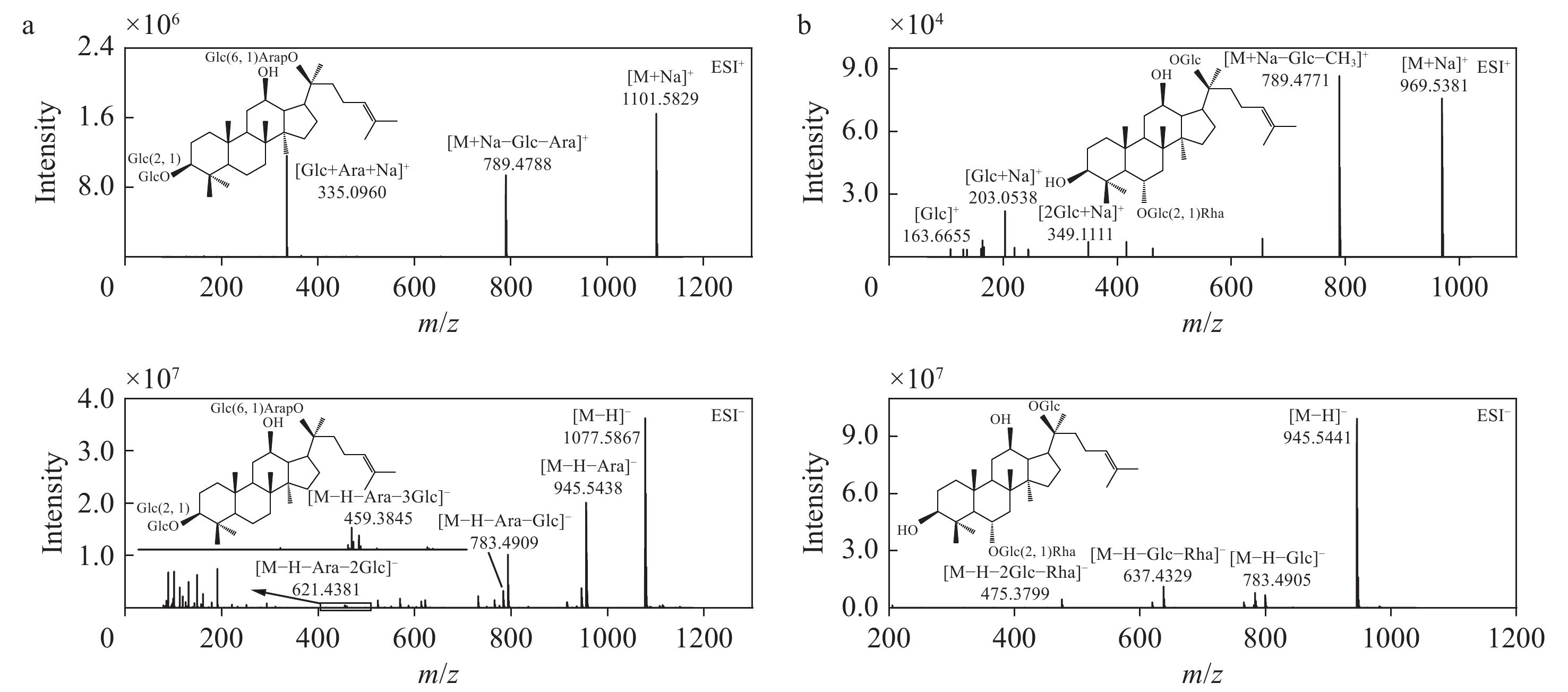
以人参皂苷Rc为例分析PPD型人参皂苷的主要裂解途径,其二级质谱图示于图3a。在负离子模式下,一级质谱出现准分子离子峰m/z
1123.5914 [M+HCOO]−和1077.5867 [M−H]−,在二级质谱中,C-20位上丢失1分子阿拉伯糖残基生成m/z945.5438 [M−H−Ara]−,丢失1分子阿拉伯糖残基和1分子葡萄糖残基生成m/z783.4909 [M−H−Ara−Glc]−。m/z621.4381 [M−H−Ara−2Glc]−和459.3845 [M−H−Ara−3Glc]−碎片离子是C-20、C-3位上丢失葡萄糖和阿拉伯糖产生的。此外,还检测到一些糖链末端交叉环断裂生成的碎片离子,如m/z 221对应的Glc−Glc末端的交叉环断裂。正离子模式下,人参皂苷Rc一级质谱出现准分子离子峰m/z 1101.5829 [M+Na]+,m/z789.4788 和m/z335.0960 作为1对互补离子,分别代表脱去C-20位上1分子葡萄糖和1分子阿拉伯糖的[M+Na−Glc−Ara]+碎片离子和二糖的钠加合离子。PPT型人参皂苷Re的二级质谱图示于图3b。在负离子模式下,一级质谱出现准分子离子峰m/z
991.5497 [M+HCOO]−和945.5441 [M−H]−,在二级质谱中,m/z783.4905 [M−H−Glc]−碎片离子为C-20位上丢失1分子葡萄糖残基生成的,继续丢失C-6位上1分子鼠李糖残基生成m/z637.4329 [M−H−Glc−Rha]−,进而丢失1分子鼠李糖残基和1分子葡萄糖残基生成m/z475.3799 [M−H−2Glc−Rha]−。此外,在二级质谱中还检测到Glc−Rha糖链的交叉环断裂生成的m/z 205碎片离子。在正离子模式下,人参皂苷Re的一级质谱图出现准分子离子峰m/z969.5381 [M+Na]+,其失去1分子葡萄糖和1分子甲基生成m/z789.4771 [M+Na−Glc−CH3]+碎片离子,m/z349.1111 [2Glc+Na]+和203.0538 [Glc+Na]+为葡萄糖的钠加合离子。 -
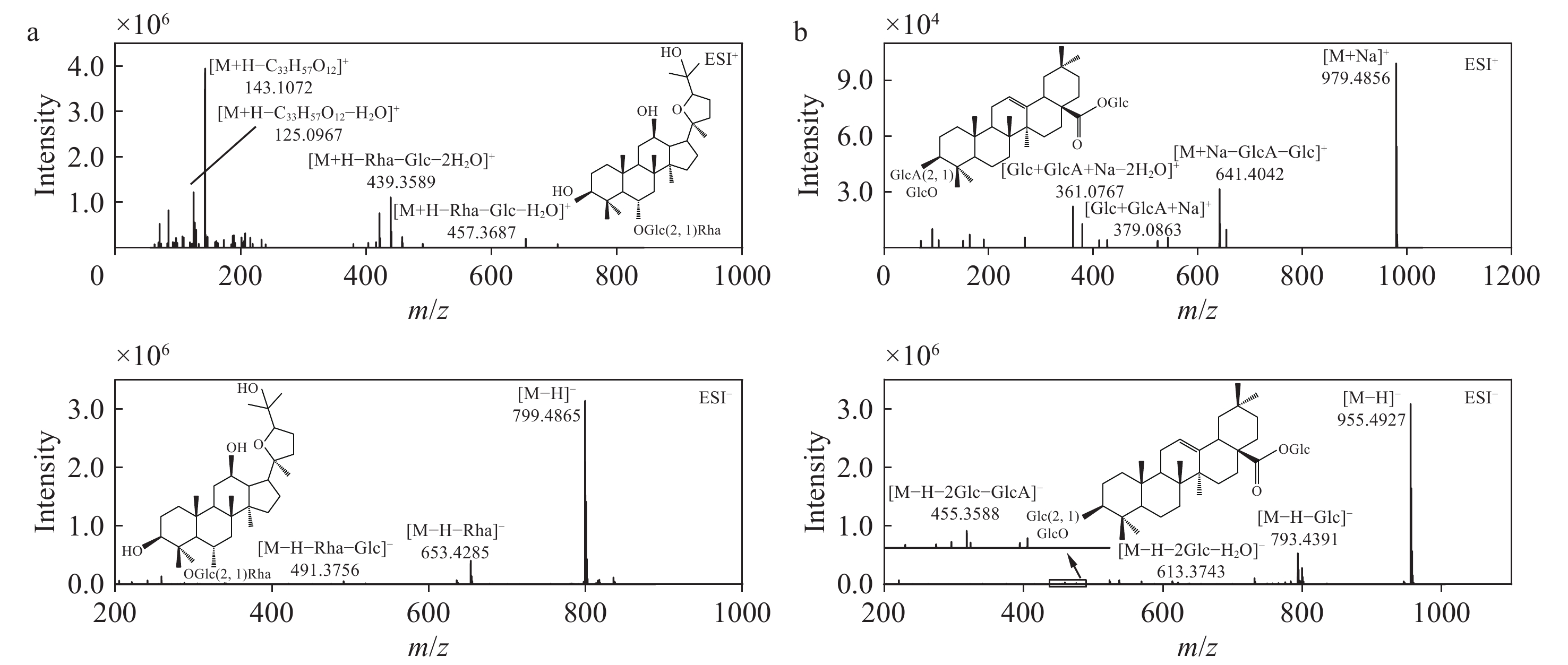
OT型人参皂苷是西洋参区别于其他人参属药用植物的特征成分,拟人参皂苷F11是其典型代表,二级质谱图示于图4a。负离子模式下,一级质谱图中出现准分子离子峰m/z
845.4883 [M+HCOO]−和799.4865 [M−H]−,二级质谱图中m/z799.4865 [M−H]−离子丢失C-6位上1分子鼠李糖残基生成m/z653.4285 [M−H−Rha]−碎片离子,丢失C-6位上1分子鼠李糖残基和1分子葡萄糖生成m/z491.3756 [M−H−Rha−Glc]−碎片离子,该离子为OT型皂苷苷元的特征碎片离子。正离子模式下,拟人参皂苷F11的一级质谱图中出现准分子离子峰m/z801.5019 [M+H]+,二级质谱图中,由于Glc、Rha和水分子的丢失,产生m/z457.3687 [M+H−Rha−Glc−H2O]+和m/z439.3589 [M+H−Rha−Glc−2H2O]+碎片离子,m/z143.1072 [M+H−C33H57O12]+和125.0967 [M+H−C33H57O12−H2O]+为侧链断裂产生的碎片离子。人参皂苷Ro作为OA型人参皂苷,在西洋参和人参中含量丰富,其二级质谱图示于图4b。负离子模式下,一级质谱出现准分子离子峰m/z
955.4927 [M−H]−,二级质谱中,由于Glc、Glc、GlcA(葡萄糖醛酸)和水分子的丢失,产生了典型的碎片离子m/z793.4391 [M−H−Glc]−、 m/z613.3743 [M−H−2Glc−H2O]−和m/z455.3588 [M−H−2Glc−GlcA]−,其中m/z455.3588 为OA型皂苷苷元的特征碎片离子。正离子模式下,一级质谱出现准分子离子峰m/z979.4856 [M+Na]+,m/z641.4042 和m/z379.0863 作为1对互补离子,分别为脱去1分子葡萄糖和1分子葡萄糖醛酸的[M+Na−Glc−GlcA]+碎片离子和二糖的钠加合离子[Glc+GlcA+Na]+,m/z361.0767 为[Glc+GlcA+Na−2H2O]+碎片离子。 -
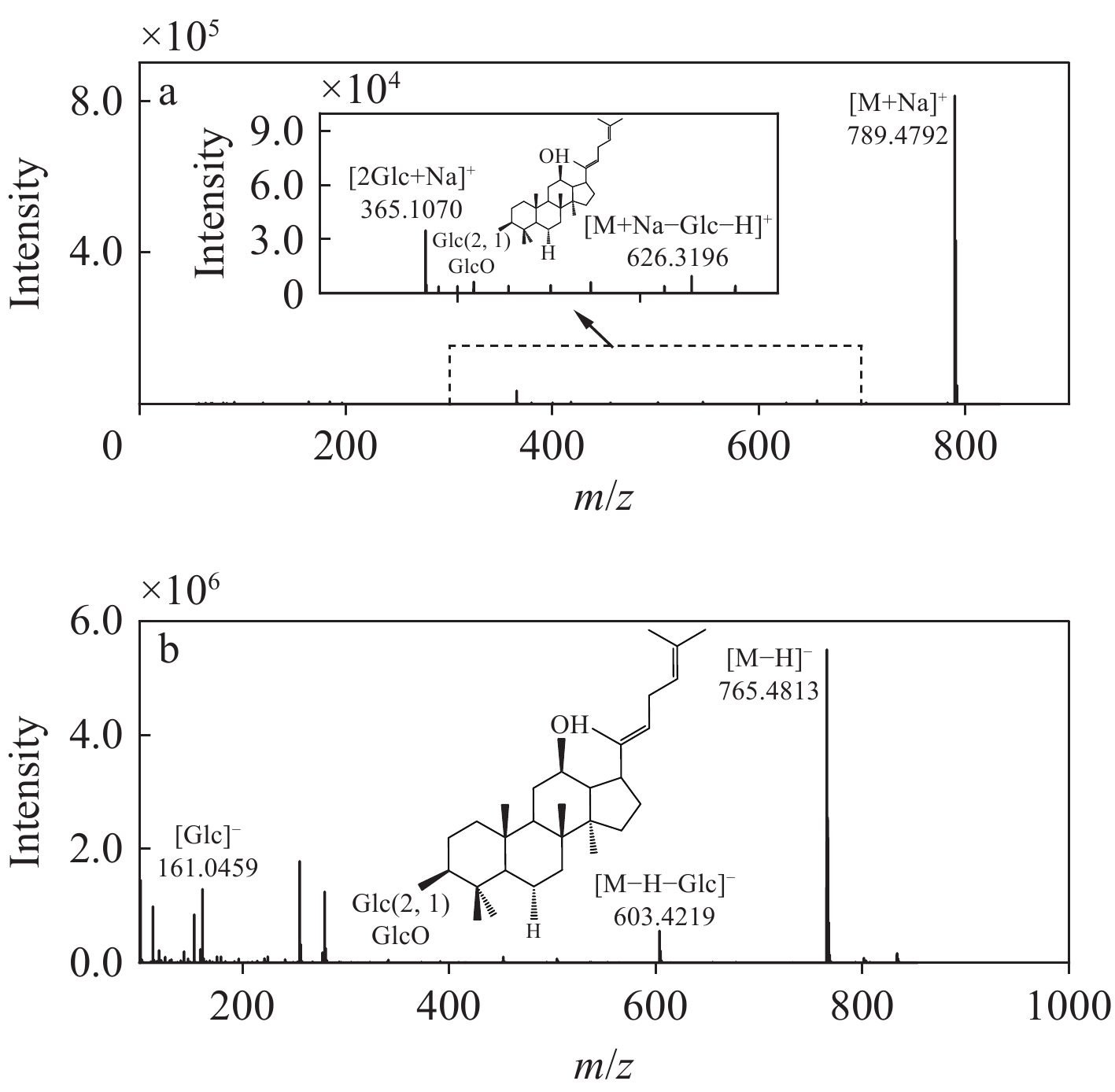
以人参皂苷Rg5为例分析裂解规律,其二级质谱图示于图5。负离子模式下,一级质谱图出现准分子离子峰m/z 811.486 2[M+HCOO]−,二级质谱中m/z
765.4813 为[M−H]−离子,丢失1分子葡萄糖残基生成m/z603.4219 [M−H−Glc]−碎片离子,此外,还出现m/z161.0459 [Glc]−碎片离子。正离子模式下,人参皂苷Rg5一级质谱出现准分子离子峰m/z789.4792 [M+Na]+,m/z626.3196 为[M+Na−Glc−H]+碎片离子,m/z365.1070 为二糖的钠加合离子[2Glc+Na]+。 -
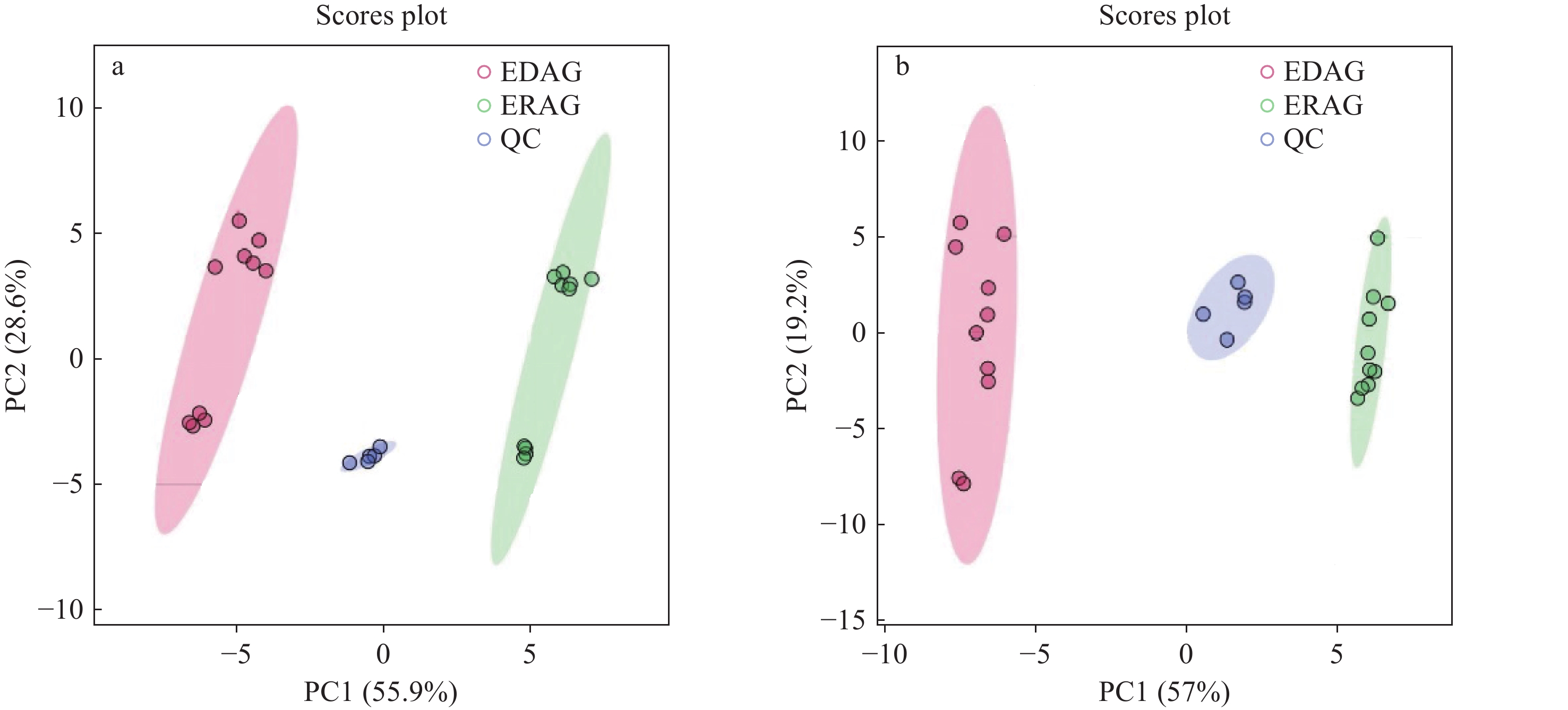
在正、负离子模式下,生晒西洋参和红西洋参的PCA得分图示于图6。可见,2组样本完全分离,表明生晒西洋参和红西洋参之间存在显著差异;QC样本相对集中,表明仪器稳定性良好。
-
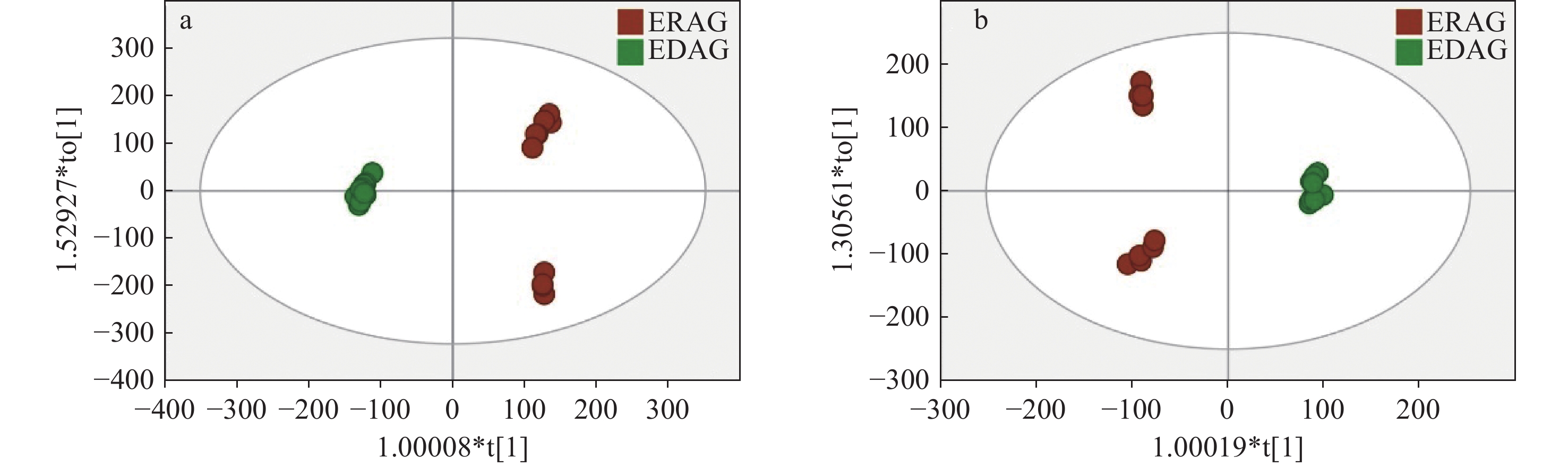
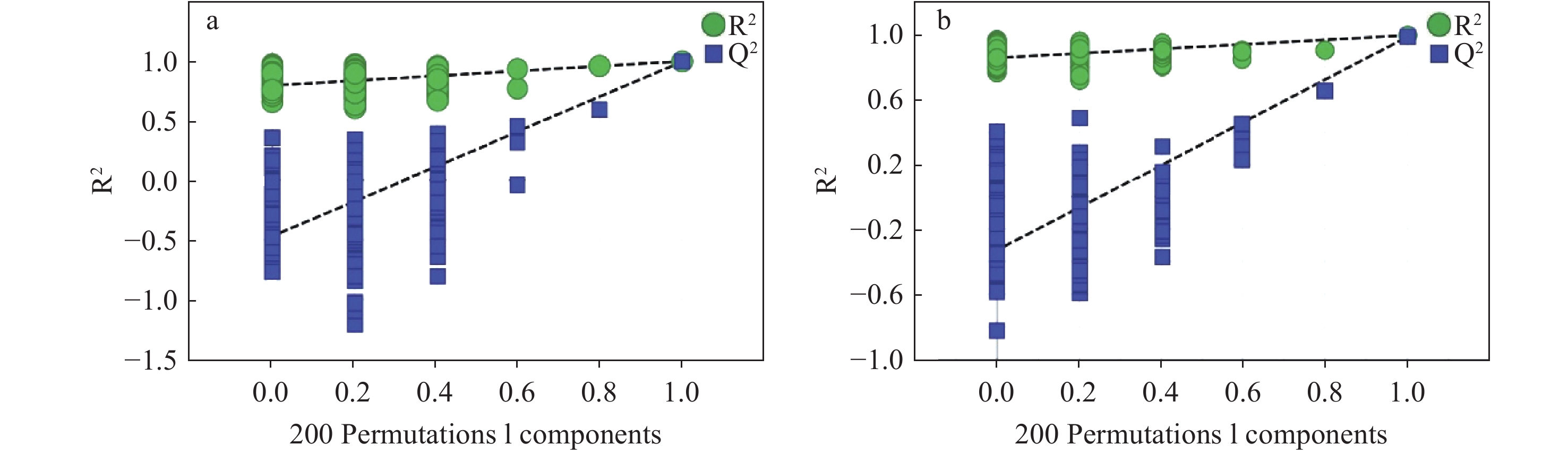
为进一步分析2组间化合物的差异信息,对2组数据进行有监督的OPLS-DA,结果示于图7。在正、负离子模式下,生晒西洋参和红西洋参均分离良好,分布在左右两侧,表明两者存在显著差异。OPLS-DA模型参数显示,正离子模式下,R2Y为0.997,Q2为0.992,负离子模式下,R2Y为0.996,Q2为0.986,表明所建立的模型具有良好的拟合度和较高的可预测性。置换检验(n=200)结果示于图8,Q2回归线在Y轴上的截距均小于0,模型稳定,未出现过拟合。
-
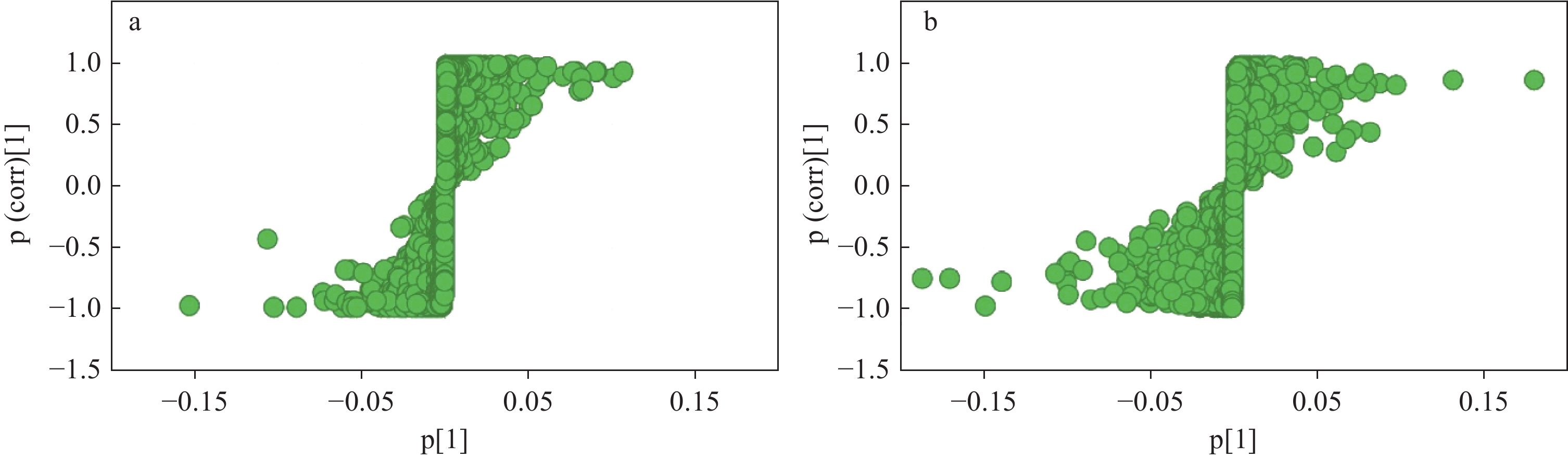
正、负离子模式下,生晒西洋参和红西洋参的S-plot图示于图9,化合物越靠近右上角和左下角,表示其对样本分类贡献越大。保留VIP>1的化合物作为候选差异化合物,根据student-t检验结果,进一步筛选出P<0.05的化合物作为能够区分生晒西洋参与红西洋参的差异化合物。将保留时间、质荷比、碎片离子信息与多元统计学分析结果相匹配,对生晒西洋参和红西洋参中的差异性皂苷进行表征。通过筛选鉴定得到31种差异性皂苷,包括14种PPD型,11种PPT型,6种其他型。
-
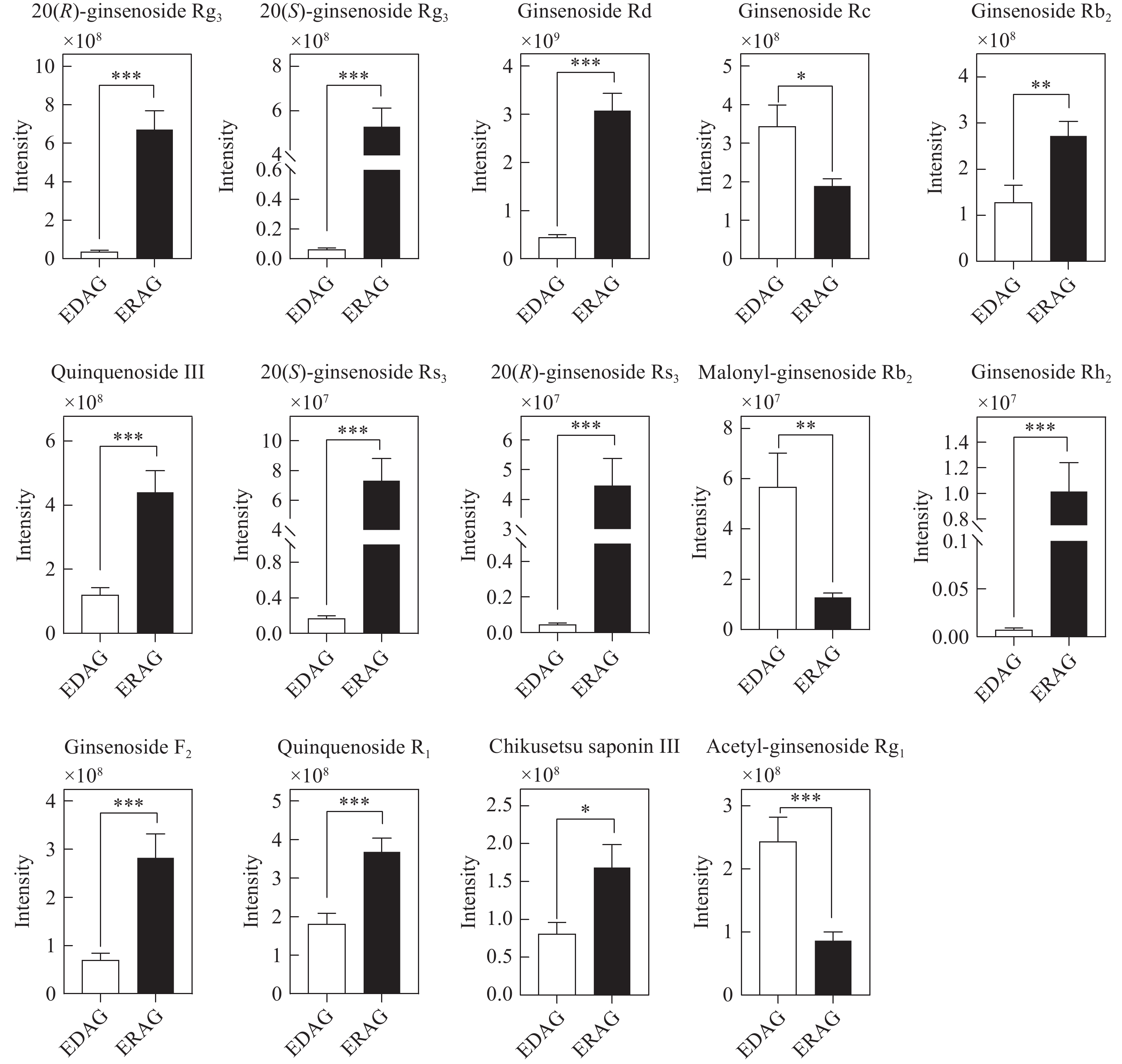
生晒西洋参和红西洋参中原人参二醇型差异皂苷相对含量柱形图示于图10。在筛选鉴定到的14种PPD型差异性皂苷中,人参皂苷Rc、丙二酰人参皂苷Rb2和乙酰人参皂苷Rg1在生晒西洋参中含量较高;11种人参皂苷在红西洋参中含量较高,包括人参皂苷Rd、Rb2等原型皂苷和部分稀有皂苷(人参皂苷Rg3、Rs3、Rh2等)。
-
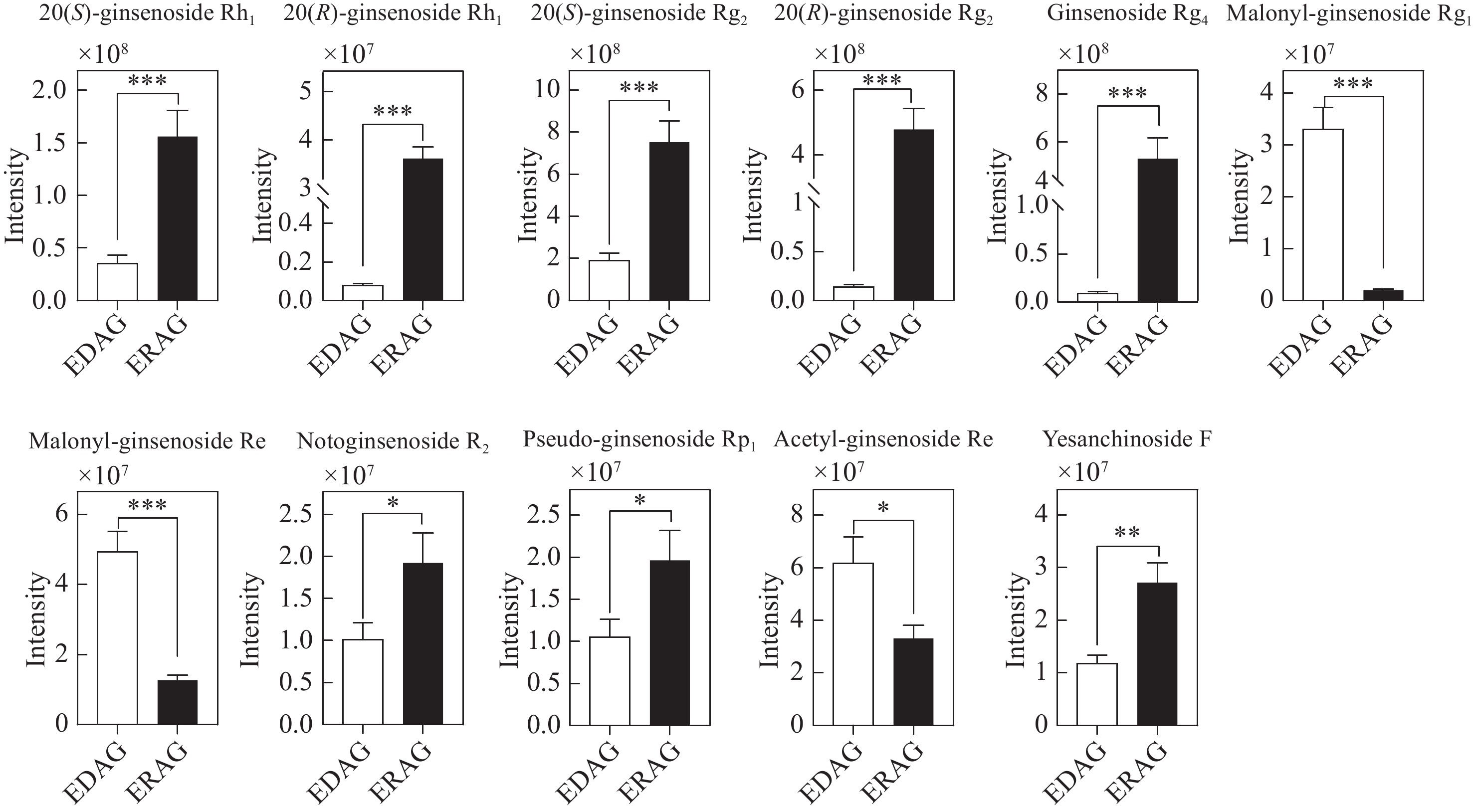
生晒西洋参和红西洋参中原人参三醇型差异皂苷相对含量柱形图示于图11。在筛选鉴定到的11种PPT型差异性皂苷中,丙二酰人参皂苷Rg1、丙二酰人参皂苷Re和乙酰人参皂苷Re在生晒西洋参中含量较高,8种人参皂苷在红西洋参中含量较高,包括人参皂苷Rg2、西洋参皂苷R2等原型皂苷和部分稀有皂苷。
-
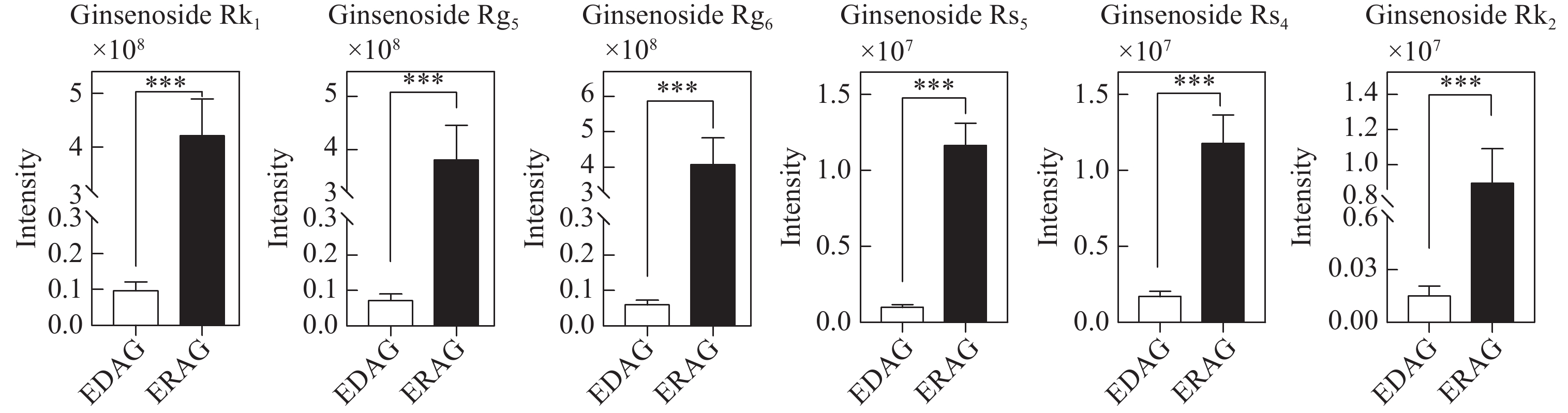
本研究筛选鉴定到的6种其他类型人参皂苷均为稀有皂苷,且均在红西洋参中含量更高,示于图12。
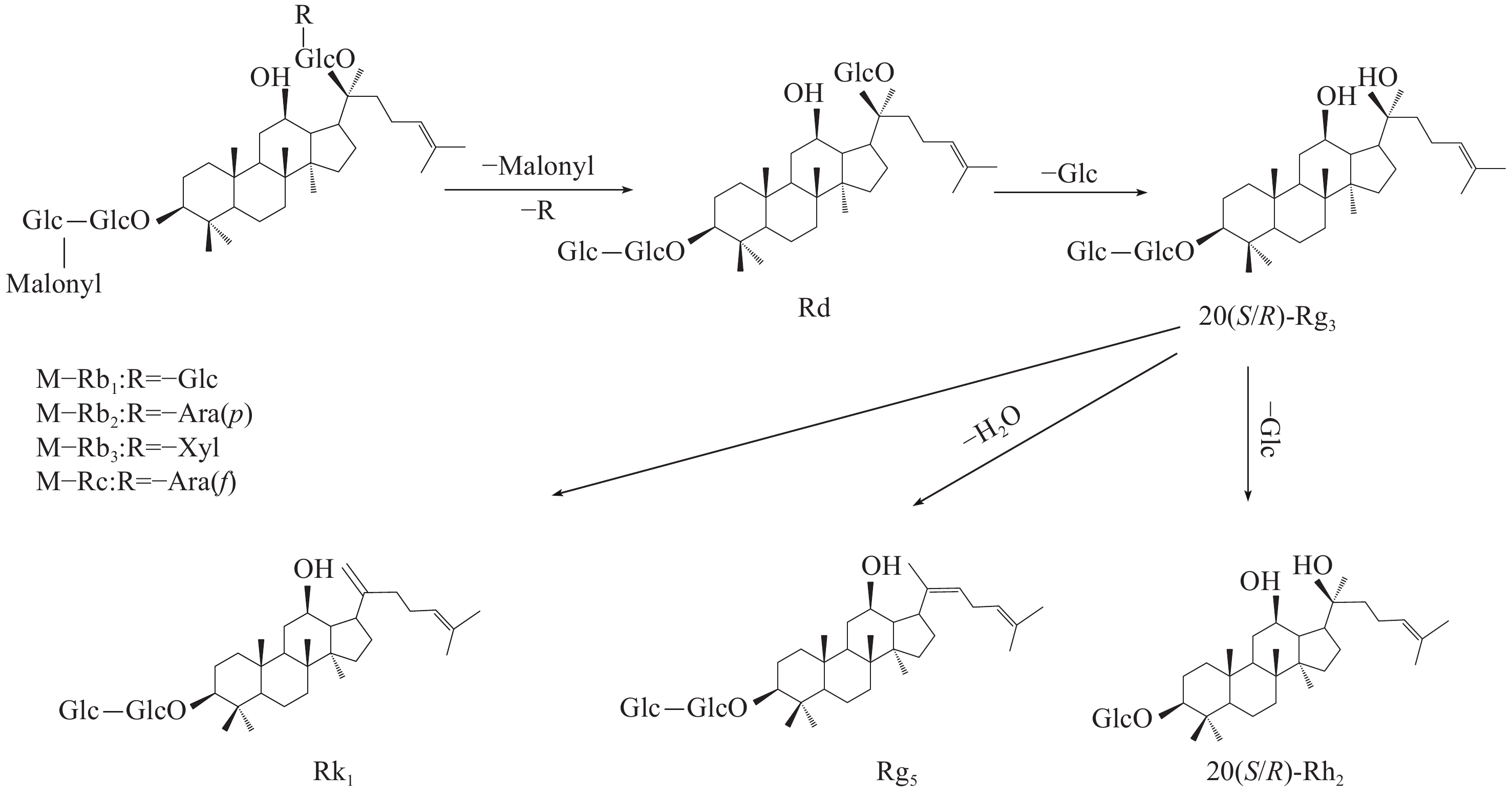
综上所述,乙酰基和丙二酰基取代的人参皂苷在生晒西洋参中含量更高,大部分稀有皂苷在红西洋参中含量更高,造成这种差异的原因是在红西洋参加工过程中,由于温度的影响,水解酶、淀粉酶、麦芽酶等被破坏,阻止皂苷水解,加速了原型皂苷的生物转化[15-19],特别是C-20位上的糖基易发生水解和去糖基化,然后发生异构化或脱水,部分原型皂苷转化为稀有皂苷[20]。此外,高温促进脱乙酰基和去丙二酰基等化学反应。丙二酰人参皂苷Rb2和Rc等可通过脱去C-3位上的丙二酰基和C-20位上的糖基转化为人参皂苷Rg3;人参皂苷Rg3可通过脱去C-3位上1分子葡萄糖转化为人参皂苷Rh2,也可脱去1分子水转化为人参皂苷Rk1和Rg5等稀有皂苷,转化过程示于图13。
-
本研究采用UHPLC-Q-Orbitrap-MS/MS技术对生晒西洋参和红西洋参中66种皂苷类成分进行表征,总结正、负离子模式下4种典型皂苷的裂解规律,并通过OPLS-DA模型筛选出生晒西洋参和红西洋参中31种差异性皂苷,包括14种PPD型,11种PPT型,6种其他型。本研究可为西洋参中皂苷的发现提供技术支持,同时为西洋参资源的开发提供理论依据。
基于UHPLC-Q-Orbitrap-MS/MS分析2种西洋参炮制品中皂苷类成分
Analysis of Saponins in Two Processed American Ginseng Products Based on UHPLC-Q-Orbitrap-MS/MS
-
摘要: 采用超高效液相色谱-四极杆-静电场轨道阱高分辨质谱(UHPLC-Q-Orbitrap-MS/MS)技术对生晒西洋参和红西洋参中皂苷类成分进行分析,并结合主成分分析、正交偏最小二乘法判别分析和差异成分分析比较2种西洋参炮制品中差异性皂苷。采用Supelco C18色谱柱(3.0 mm×50 mm,2.7 μm),以0.1%甲酸水溶液-乙腈作为流动相进行梯度洗脱,在电喷雾离子源正、负离子模式下进行全扫描和二级质谱扫描。本实验鉴定了生晒西洋参和红西洋参中66种皂苷类成分,其中包括31种差异性皂苷。基于实验结果,通过总结4种类型皂苷的质谱碎裂规律,列举了部分皂苷可能的转化过程。该方法准确、高效,可为西洋参及其炮制品的药效物质基础研究和质量评价提供理论依据。Abstract: Ultra-high performance liquid chromatography-quadrupole-electrostatic field orbital trap high-resolution mass spectrometry (UHPLC-Q-Orbitrap-MS/MS) was used to analyze the saponin components in sun-dried American ginseng and red American ginseng. The principal component analysis, orthogonal partial least squares discriminant analysis, and differential component analysis were employed to compare the differential saponins in these two processed products of ginseng. A Supelco C18 column (3.0 mm×50 mm, 2.7 μm) was used with 0.1% formic acid in water and acetonitrile as the mobile phase for gradient elution. Full primary MS scan and secondary MS scan were performed by an electrospray ionization source under both positive and negative ion modes. A total of 66 saponin components are identified in sun-dried American ginseng and red American ginseng, among which 31 are differential saponins, including 14 PPD-type, 11 PPT-type, and 6 other types. Among the 14 PPD-type differential saponins identified by screening, 3 ginsenosides are higher in sun-dried American ginseng, including ginsenoside Rc, malonyl ginsenoside Rb2, and acetyl ginsenoside Rg1, while 11 ginsenosides are higher in red American ginseng, including prototypical saponins, such as ginsenoside Rd, ginsenoside Rb2, and some rare saponins (ginsenoside Rg3, ginsenoside Rs3, ginsenoside Rh2, and others). Among the 11 PPT-type differential saponins, 3 ginsenosides are found with high content in sun-dried American ginseng, including malonyl ginsenoside Rg1, malonyl ginsenoside Re and acetyl ginsenoside Re, while 8 ginsenosides are found with high content in red American ginseng, including prototypic saponins, such as ginsenoside Rg2, American ginsenoside R2 and some rare saponins (ginsenoside Rh1, ginsenoside Rg4). Additionally, 6 other types of ginsenosides, all of which are rare saponins and more abundant in red ginseng. Acetyl- and malonyl-substituted ginsenosides are more abundant in sun-dried American ginseng, while most of the rare saponins are more abundant in red American ginseng. Based on the results of the tandem MS scans of the identified compounds, the mass spectrometric fragmentation pathways of the four types of saponins were summarized, and the possible transformation processes of some saponins were enumerated. Due to the effect of temperature, the sugar group at the C-20 position is prone to hydrolysis and deglycosylation, followed by isomerization or dehydration. Some of the prototypical saponins are converted into rare saponins, in addition to high temperature promoted chemical reactions, such as deacetylation and depropanediylation. This method is accurate and efficient, and can provide a theoretical basis for the pharmacological material basis and quality evaluation of American ginseng.
-
Key words:
- high resolution mass spectrometry /
- sun-dried American ginseng /
- red American ginseng /
- saponins .
-

-
表 1 生晒西洋参和红西洋参中66种人参皂苷成分信息
Table 1. Chemical composition information of 66 kinds of ginsenosides in sun-dried American ginseng and red American ginseng
序号
No.化合物名称
Compound
name保留时间
tR/min分子式
Molecular
formula理论分子质量
Theoretical
molecular
weight加合形式
Calculated massMS/MS碎片离子
Fragment ion (m/z)类型
TypeESI−
m/z
(误差/×10−6)ESI+
m/z
(误差/×10−6)ESI− ESI+ 1 三七皂苷ST5 4.56 C47H80O18 932.5345 977.5334b
(1.86)955.5226c
(−1.10)931.5280,
799.4772ND PPD 2 人参皂苷Re1/Re2/Re3 7.57 C48H82O19 962.5450 1007.5482b
(5.97)985.5362c
(1.97)799.4854,
637.4335,
475.3801805.4760 PPT 3 西洋参皂苷F6 7.82 C47H80O18 932.5345 977.5338b
(2.29)955.5280c
(4.53)931.5286,
799.4862,
637.4338335.0962 PPT 4 三七皂苷 R1 9.23 C47H80O18 932.5345 977.5341b
(2.25)955.5234c
(−0.26)931.5278,
799.4800,
637.2721755.4648,
498.6733,
335.0963PPT 5 西洋参皂苷 Ⅳ 12.23 C54H90O24 1122.5822 1167.5837b
(3.37)1145.5714c
(−0.01)1121.5767,
959.5218,
797.4675803.4565,
365.1081PPD 6 人参皂苷Rg1* 12.67 C42H72O14 800.4922 845.4899b
(0.73)823.4806c
(−1.02)799.4868,
637.4335,
475.3806643.4205,
543.0381,
415.7682,
385.9017,
203.0533PPT 7 人参皂苷 Re* 13.44 C48H82O18 946.5501 991.5497b
(2.48)969.5381c
(−1.29)945.5441,
783.4905,
637.4329,
475.3799789.4771,
462.3381,
415.7650,
349.1111,
203.0538,
163.6655PPT 8 西洋参皂苷L11 15.83 C42H72O14 800.4922 845.4918b
(2.97)801.5035d
(5.07)799.4860,
653.4277,
491.3752783.4868,
457.3693,
143.1072OT 9 丙二酰人参皂苷Rg1 16.73 C45H74O17 886.4926 885.4900a
(5.27)909.4856c
(4.16)799.4861,
637.4329,
475.3797729.4198,
685.4243,
415.7838,
203.0533PPT 10 丙二酰人参皂苷Re 17.05 C51H84O21 1032.5505 1031.5488a
(5.43)1055.5402c
(0.41)945.5393,
637.3092,
475.2528,
637.3076875.4814 PPT 11 人参皂苷 F5 17.62 C41H70O13 770.4816 815.4802b
(1.73)ND 769.4763,
637.4321,
475.3821ND PPT 12 西洋参皂苷 L14 17.87 C47H80O17 916.5396 961.5415b
(5.03)ND 915.5330,
783.4910,
799.4828ND PPD 13 乙酰人参皂苷 Rg1 17.95 C44H74O15 842.5028 887.5034b
(3.39)865.4952c
(3.69)841.4962,
637.4326,
475.3801415.8132,
163.6590PPD 14 珠子参苷R1 18.14 C42H72O15 816.4871 861.4855b
(1.44)ND 815.4813,
491.3762ND OT 15 乙酰人参皂苷 Re 18.18 C50H84O19 988.5607 1033.5627b
(4.79)ND 987.5535,
945.5436,
783.4848,
621.4312ND PPT 16 珠子参苷 F5 18.37 C48H82O19 962.5450 1007.5471b
(4.94)985.5385c
(4.26)961.5370,
815.4803,
799.4754,
653.3394805.4730 PPD 17 珠子参苷F1 18.82 C48H82O19 962.5450 1007.5472b
(5.06)985.5392c
(5.00)961.5378,
637.3096805.4749 PPD 18 珠子参苷R2 19.09 C41H70O14 786.4766 831.4728b
(−0.99)809.4670c
(1.56)785.4708,
653.4283,
491.3757567.4276 OT 19 拟人参皂苷 F11* 19.47 C42H72O14 800.4922 845.4883b
(−1.22)801.5019d
(3.01)799.4865,
653.4285,
491.3756457.3687,
439.3589,
143.1072,
125.0967OT 20 人参皂苷Ra3 19.79 C59H100O27 1240.6452 1285.6447b
(1.83)ND 1239.6400,
1107.5972,
783.4885,
621.4404,
459.3879ND PPD 21 三七皂苷 R2 20.08 C41H70O13 770.4816 815.4817b
(3.61)ND 769.4758,
637.4335,
475.3796ND PPT 22 三七皂苷 Fa 20.31 C59H100O27 1240.6452 1285.6447b
(1.83)1263.6364c
(1.53)1239.6396,
1107.5961,
945.5437,
783.4904,
459.3881497.1496,
335.0959PPD 23 人参皂苷 F3 20.79 C41H70O13 770.4816 815.4785b
(−0.28)793.4725c
(2.03)637.4329,
475.3797335.0960 PPT 24 西洋参皂苷 L6 20.82 C48H80O18 944.5345 989.5327b
(1.09)ND 943.5281,
781.4756,
619.4228,
457.3706ND PPD 25 20(S)-人参皂苷 Rg2 21.10 C42H72O13 784.4973 829.4966b
(2.70)807.4859c
(−0.76)783.4906,
637.4335,
475.3796,
459.3859543.0711,
458.1806,
349.1121,
204.0869PPT 26 20(S)-人参皂苷Rh1* 21.19 C36H62O9 638.4394 683.4368b
(0.50)ND 637.4329,
475.3793ND PPT 27 20(R)-人参皂苷Rg2* 21.46 C42H72O13 784.4973 829.4946b
(0.28)807.4863c
(−0.30)783.4906,
637.4335,
475.3796,
459.3859726.0026,
543.0608,
415.7874,
349.1113PPT 28 20(R)-人参皂苷Rh1* 21.85 C36H62O9 638.4394 683.4388b
(3.44)ND 637.4324,
475.3793ND PPT 29 人参皂苷Rb1* 22.03 C54H92O23 1108.6029 1153.6027b
(2.27)1131.5933c
(0.97)1107.5975,
945.5433,
621.4377,
459.3855789.4785,
365.1067PPD 30 人参皂苷 Rc 22.90 C53H90O22 1078.5924 1123.5914b
(1.74)1101.5829c
(1.17)1077.5867,
945.5438,
783.4909,
621.4381,
459.3845789.4788,
335.0960PPD 31 人参皂苷 Ro 22.95 C48H76O19 956.4981 955.4927a
(1.97)979.4856c
(−1.74)793.4391,
613.3743,
569.3880,
455.3588641.4042,
379.0863,
361.0767OA 32 丙二酰人参皂苷Rb1 23.21 C57H94O26 1194.6033 1193.5964a
(0.32)1217.5989c
(5.20)1107.5963,
945.5438,
783.4908,
621.4366789.4747,
451.1075PPD 33 丙二酰人参皂苷Rb3 23.67 C56H92O25 1164.5928 1163.5911a
(4.81)1187.5830c
(0.86)1077.5863,
945.5436,
783.4908,
621.43801143.5768,
875.4791,
789.4797,
335.0960PPD 34 人参皂苷 Rb3 24.07 C53H90O22 1078.5924 1123.5942b
(4.24)1101.5877c
(5.49)1077.5856,
945.5426,
783.4324,
621.3091,
459.1932789.4786,
509.1430,
335.0961PPD 35 人参皂苷 Rb2 24.60 C53H90O22 1078.5924 1123.5931b
(3.26)1101.5823c
(0.62)1077.5858,
945.5091,
783.4170,
621.8104789.4790,
335.0961PPD 36 丙二酰人参皂苷Rb2 25.16 C56H92O25 1164.5928 1163.5924a
(5.95)ND 1077.5865,
945.5439,
783.4908,
621.4379ND PPD 37 拟人参皂苷 Rt1 25.38 C47H74O18 926.4875 925.4824a
(2.29)949.4771c
(0.33)793.4405,
763.4315641.4077,
331.0646OA 38 丙二酰人参皂苷Rc 25.77 C56H92O25 1164.5928 1163.5894a
(3.35)ND 1119.5964,
1077.5863,
945.5417,
783.4899ND PPD 39 西洋参皂苷R1 26.13 C56H94O24 1150.6135 1195.6134b
(2.33)1173.6050c
(1.93)1149.6074,
1107.5969,
987.5587,
945.5435,
783.4909831.4890,
365.1068PPD 40 竹节参皂苷Iva 26.96 C42H66O14 794.4453 793.4425a
(5.70)817.4377c
(3.99)631.3857,
613.3774,
569.3871,
455.3549641.3996 OA 41 人参皂苷Rd* 27.72 C48H82O18 946.5501 991.5499b
(2.73)969.5399c
(0.60)945.5444,
783.4909,
621.4381,
459.3856789.4791,
365.1070PPD 42 乙酰人参皂苷 Fc 28.12 C55H92O23 1120.6029 1165.6003b
(0.26)1143.5956c
(2.99)1077.5861,
945.5433,
783.4906,
621.4382831.4890,
335.0961PPD 43 人参皂苷Rs1/Rs2 28.73 C55H92O23 1120.6029 1119.5966a
(0.80)ND 1077.5962,
945.5447,
783.4910,
621.4382ND PPD 44 丙二酰人参皂苷Rd 28.95 C51H84O21 1032.5505 1031.5447a
(1.41)1055.5436c
(3.65)987.5536,
945.5439,
783.4908,
621.4379,
459.38601011.5564,
875.4794,
831.4880PPD 45 野三七皂苷F 30.63 C56H94O24 1150.6135 1195.6130b
(2.03)1173.6054c
(2.24)1107.5968,
1089.5859,
927.5335,
825.5055,
793.4407,
781.4738,
663.4514831.4891,
365.1068PPT 46 PPD-Glc-(Glc-Glc)-2malonyl 31.74 C54H86O24 1118.5509 1117.5472a
(3.23)1141.5454c
(4.63)945.5442,
783.4901,
621.4377,
459.3857875.4787,
831.4874Other 47 竹节参皂苷Ⅲ 33.63 C47H80O17 916.5396 961.5367b
(0.02)939.5314c
(2.83)915.5336,
783.4921,
621.4384,
459.3856335.0960 PPD 48 乙酰人参皂苷 Fe 34.27 C47H80O17 916.5396 961.5388b
(2.18)939.5337c
(5.24)915.5345,
783.4912,
621.4384789.4823,
415.9040PPD 49 西洋参皂苷 Ⅲ 34.44 C50H84O19 988.5607 1033.5609b
(3.02)1011.5552c
(5.22)987.5546,
945.5442,
783.4908,
621.4387,
459.3867831.4900,
598.4606,
415.9045PPD 50 绞股蓝皂苷 IX 34.80 C47H80O17 916.5396 961.5382b
(1.61)939.5339c
(5.43)961.5371,
783.4907,
621.4388789.4823,
335.0962PPD 51 人参皂苷 Rg6 35.14 C42H70O12 766.4867 811.4868b
(3.68)789.4797c
(4.80)765.4771,
619.3341,
457.2831,
654.8112543.3770,
488.2852,
416.0153,
365.1076Other 52 人参皂苷 Rg4 35.89 C42H70O12 766.4867 811.4871b
(3.98)789.4759c
(−0.08)765.4808,
619.4227,
457.3726349.1119 PPT 53 绞股蓝皂苷 X 36.57 C48H82O17 930.5552 975.5533b
(1.06)953.5494c
(5.26)929.5496,
783.4897,
767.4971,
621.4386,
459.3862687.2725,
543.1977,
163.6961PPD 54 人参皂苷 F2 36.88 C42H72O13 784.4973 829.4948b
(0.42)807.4857c
(−0.98)783.4890,
621.4366627.4250,
451.2794,
203.0534PPD 55 竹节参皂苷 Ib 37.51 C47H74O18 926.4875 925.4796a
(−0.75)ND 793.4396,
731.4383,
569.3860,
455.3539ND OA 56 20(R)-人参皂苷 Rg3* 38.51 C42H72O13 784.4973 829.4952b
(0.96)807.4855c
(−1.21)783.4913,
621.4379,
459.3858545.2290,
365.1067PPD 57 20(S)-人参皂苷 Rg3* 38.84 C42H72O13 784.4973 829.4959b
(1.81)807.4904c
(4.84)783.4915,
621.4383,
459.3857415.9317,
365.1083PPD 58 拟人参皂苷 Rp1 39.79 C41H64O13 764.4347 763.4320a
(5.93)ND 631.3867,
613.3767,
469.3870ND PPT 59 20(S)-人参皂苷 Rs3 40.80 C44H74O14 826.5079 871.5085b
(4.04)849.4991c
(2.35)783.4913,
621.4383,
459.3855746.0770,
543.3107PPD 60 20(R)-人参皂苷 Rs3 41.10 C44H74O14 826.5079 871.5079b
(3.41)849.4993c
(2.58)783.4846,
621.1266,
459.2041768.5690 PPD 61 人参皂苷Rk1* 42.22 C42H70O12 766.4867 811.4867b
(3.52)767.4935d
(−0.69)765.4813,
603.4219,
161.0459543.3455,
425.3788,
407.3678Other 62 人参皂苷Rg5* 42.61 C42H70O12 766.4867 811.4862b
(2.92)789.4792c
(4.10)765.4813,
603.4219,
161.0459626.3196,
365.1070Other 63 人参皂苷 Rs5 42.68 C44H72O13 808.4973 807.4918a
(2.23)ND 765.4807,
603.4272ND Other 64 人参皂苷 Rs4 43.08 C44H72O13 808.4973 807.4932a
(3.98)ND 765.4818,
853.4967,
603.3398ND Other 65 人参皂苷Rh2* 43.53 C36H62O8 622.4445 667.4457b
(6.25)ND 621.4360,
459.3851,
304.9165ND PPD 66 人参皂苷 Rk2 47.56 C36H60O7 604.4339 649.4343b
(5.02)ND 161.0464 ND Other 注:*表示对照品验证的成分;a、b、c、d分别代表[M−H]−、[M+HCOO]−、[M+Na]+、[M+H]+加合形式 -
[1] 国家药典委员会. 中华人民共和国药典-一部[M]. 2020年版. 北京: 中国医药科技出版社, 2020. [2] PANG S, PIAO X, ZHANG X, CHEN X, ZHANG H, JIN Y, LI Z, WANG Y. Discrimination for geographical origin of Panax quinquefolius L. using UPLC Q-Orbitrap MS-based metabolomics approach[J]. Food Science & Nutrition, 2023, 11(8): 4 843-4 852. [3] 吴首蓉, 郭晓宇, 屠鹏飞, 姜勇. 西洋参化学成分、生物活性、品质评价及产品开发研究进展[J]. 药学学报, 2022, 57(6): 1 711-1 725. WU Shourong, GUO Xiaoyu, TU Pengfei, JIANG Yong. Research progress on chemical constituents, biological activities, quality evaluation, and product development of Panax quinquefolium[J]. Acta Pharmaceutica Sinica, 2022, 57(6): 1 711-1 725(in Chinese). [4] LIU L, XU F R, WANG Y Z. Traditional uses, chemical diversity and biological activities of Panax L. (Araliaceae): a review[J]. Journal of Ethnopharmacology, 2020, 263: 112 792. [5] PUNJA Z K. American ginseng: research developments, opportunities, and challenges[J]. J Ginseng Res, 2011, 35(3): 368 -374 . doi: 10.5142/jgr.2011.35.3.368[6] TIAN L, GAO R, CAI Y, CHEN J, DONG H, CHEN S, YANG Z, WANG Y, HUANG L, XU Z. A systematic review of ginsenoside biosynthesis, spatiotemporal distribution, and response to biotic and abiotic factors in American ginseng[J]. Food & Function, 2024, 15(5): 2 343-2 365. [7] WANG Y, CHOI H K, BRINCKMANN J A, JIANG X, HUANG L. Chemical analysis of Panax quinquefolius (north American ginseng): a review[J]. Journal of Chromatography A, 2015, 1 426: 1-15. [8] 梁力文, 郭娜, 刘小康, 黄鑫, 蔡广知, 郭云龙, 贡济宇. 基于UHPLC-Q-Orbitrap/MS的不同产地西洋参皂苷类成分分析[J]. 中成药, 2023, 45(12): 4 017-4 024. LIANG Liwen, GUO Na, LIU Xiaokang, HUANG Xin, CAI Guangzhi, GUO Yunlong, GONG Jiyu. UHPLC-Q-Orbitrap/MS-based analysis of saponins from Panacis Quinquefolii Radix of different origins[J]. Chinese Traditional Patent Medicine, 2023, 45(12): 4 017-4 024(in Chinese). [9] GUO N, BAI Y, HUANG X, LIU X, CAI G, LIU S, GUO Y, GONG J. Comparison of the saponins in three processed American ginseng products by ultra-high performance liquid chromatography-quadrupole orbitrap tandem mass spectrometry and multivariate statistical analysis[J]. International Journal of Analytical Chemistry, 2022, 2022: 6 721 937. [10] WANG Y, LI H, LI Y, ZHU H, JIN Y. Identification of natural compounds targeting Annexin A2 with an anti-cancer effect[J]. Protein & Cell, 2018, 9(6): 568 -579 .[11] 黄鑫, 李帅坪, 张勇, 刘淑莹. UPLC-MS考察西洋红参皂苷类成分对大鼠脑内神经化学物质的影响[J]. 中国实验方剂学杂志, 2017, 23(19): 111 -117 . HUANG Xin, LI Shuaiping, ZHANG Yong, LIU Shuying. Effect of saponins in red panacis quinquefolii radix on neurochemicals in rat brain by UPLC-MS[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2017, 23(19):111 -117 (in Chinese).[12] QI L, WANG C, YUAN C. Ginsenosides from American ginseng: chemical and pharmacological diversity[J]. Phytochemistry, 2011, 72(8): 689 -699 . doi: 10.1016/j.phytochem.2011.02.012[13] 张单丽, 李梦瑶, 王东升, 温馨, 李嘉欣, 刘志. 不同加热方式对丙二酰基人参皂苷降解的影响及抗氧化活性的变化[J]. 吉林农业大学学报, 2023, 45(6): 773 -780 . ZHANG Danli, LI Mengyao, WANG Dongsheng, WEN Xin, LI Jiaxin, LIU Zhi. Effects of different heating methods on degradation of malonylginsenosides and changes in antioxidant activity[J]. Journal of Jilin Agricultural University, 2023, 45(6):773 -780 (in Chinese).[14] 白敏, 毛茜, 徐金娣, 朱玲英, 朱贺, 王强, 李松林. 人参属药用植物地上部位皂苷类成分的化学和分析研究进展[J]. 中国中药杂志, 2014, 39(3): 412 -422 . BAI Min, MAO Qian, XU Jindi, ZHU Lingying, ZHU He, WANG Qiang, LI Songlin. Advance in saponins of aerial parts of Panax species[J]. China Journal of Chinese Materia Medica, 2014, 39(3):412 -422 (in Chinese).[15] 李慧芝, 赵燕芳, 王岱杰, 李慧娟, 贺吉香, 陈相峰. 基于UPLC-Q-TOF-MS/MS和MALDI-MSI的多蒸西洋参皂苷成分识别及可视化分析[J]. 中国中药杂志, 2024, 49(6): 1 526-1 539. LI Huizhi, ZHAO Yanfang, WANG Daijie, LI Huijuan, HE Jixiang, CHEN Xiangfeng. Identification and visual analysis of ginsenosides in multi-steamed roots of Panax quinquefolium based on UPLC-Q-TOF-MS/MS and MALDI-MSI[J]. China Journal of Chinese Materia Medica, 2024, 49(6): 1 526-1 539(in Chinese). [16] 林红强. 林下西洋参化学成分、抗COPD作用及新活性化合物药代动力学研究[D]. 长春: 吉林大学, 2022. [17] 司雨. 国内外西洋参营养成分及功能因子的研究[D]. 长春: 吉林大学, 2021. [18] WEI W, LIU X, TAO Y, WANG Y, GONG J, LIU S. Saponin composition comparison of black ginseng and white ginseng by liquid chromatography-mass spectrometry combined with multivariate statistical analysis[J]. Natural Product Research, 2023, 37(19): 3 297-3 301. [19] 郭佳龙, 刘畅, 王瑶, 袁成福, 袁丁, 何毓敏. 稀有人参皂苷的应用基础与开发利用研究进展[J]. 中国中药杂志, 2024, 49(2): 304 -314 . GUO Jialong, LIU Chang, WANG Yao, YUAN Chengfu, YUAN Ding, HE Yumin. Research progress on development and utilization of minor ginsenosides[J]. China Journal of Chinese Materia Medica, 2024, 49(2):304 -314 (in Chinese).[20] CHEN L, ZHANG Y, YANG X, XU J, WANG Z, SUN Y, XU W, WANG Y. Application of UPLC-triple TOF-MS/MS metabolomics strategy to reveal the dynamic changes of triterpenoid saponins during the decocting process of Asian ginseng and American ginseng[J]. Food Chemistry, 2023, 424: 136 425. -


 首页
首页 登录
登录 注册
注册



 下载:
下载: