-

Figure 1. The procedure of lipid droplet protein co-immunoprecipitation. The experimental process includes ten steps that are indicated in the flowchart. These steps can be summarized into seven key components as illustrated in the figure. They are LD isolation, treatment LD with Triton, centrifugal separation of the lipid layer and protein solution, removal of the lipid layer and collection of LD protein-containing solution, binding of LD proteins to antibody beads, washing of non-specific proteins, and elution of the bait-prey complex. The co-IP samples obtained from the experiment are used for SDS-PAGE analysis and MS-based proteomics
-
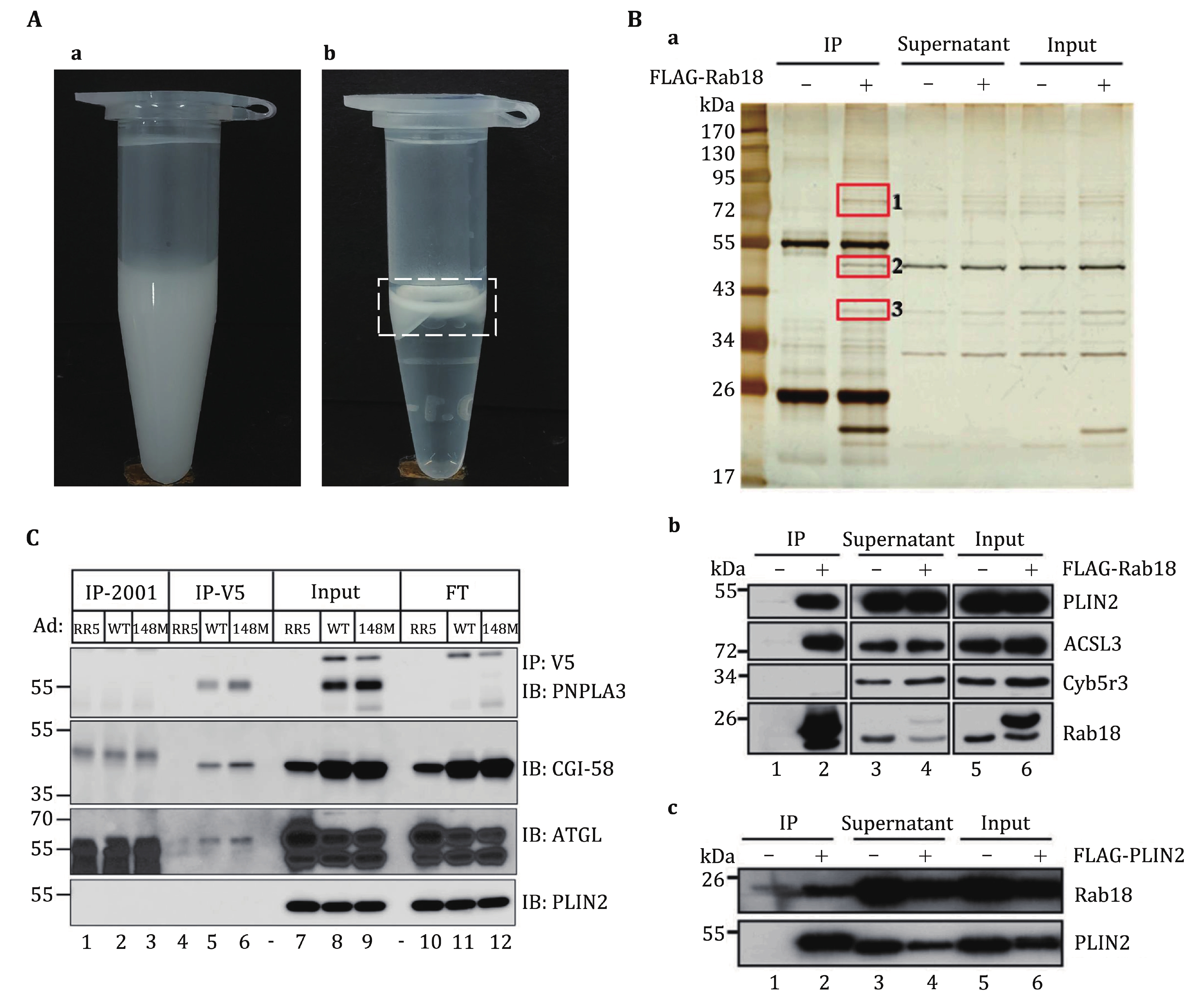
Figure 2. Anticipated results of lipid droplet co-immunoprecipitation assay. A The isolated LDs are collected in a 1.5 mL centrifuge tube. High-quality isolated LDs should present as a milky homogenate (a). The isolated LDs after RIPA1 treatment and centrifugation. The LD proteins are extracted into the solution, and after centrifugation, the LD lipids typically form a distinct top layer in the solution (b), as indicated in the white box. B LDs were isolated from OA-treated WT and FLAG-Rab18 overexpressing C2C12 cells. Immunoprecipitation was performed on the LD proteins with anti-FLAG M2 beads followed by analysis with silver staining (a). Western blot analysis of the precipitates pulled down by FLAG-Rab18 (b). Western blot analysis of the precipitates pulled down by FLAG-PLIN2 (c). The figure is reprinted from Deng et al. (2021). C Co-immunoprecipitation of PNPLA3 (WT and 148M) and CGI-58. The hepatic LDs were isolated from female mice that were infected with Ad-RR5 or Ad PNPLA3-V5 (WT or 148M). The figure is reprinted from Wang et al. (2019)
Figure
2 ,Table
2 个