-
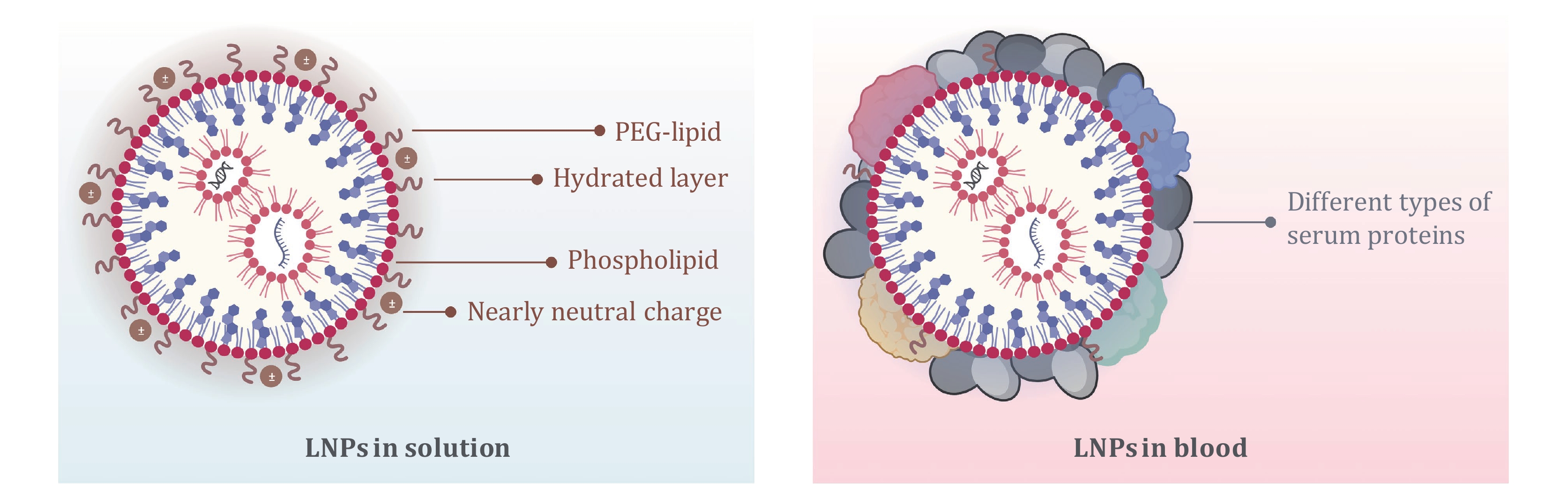
Figure 1. Different surface states of nucleic acid-LNPs in solution and in blood. Created with BioRender.com
-
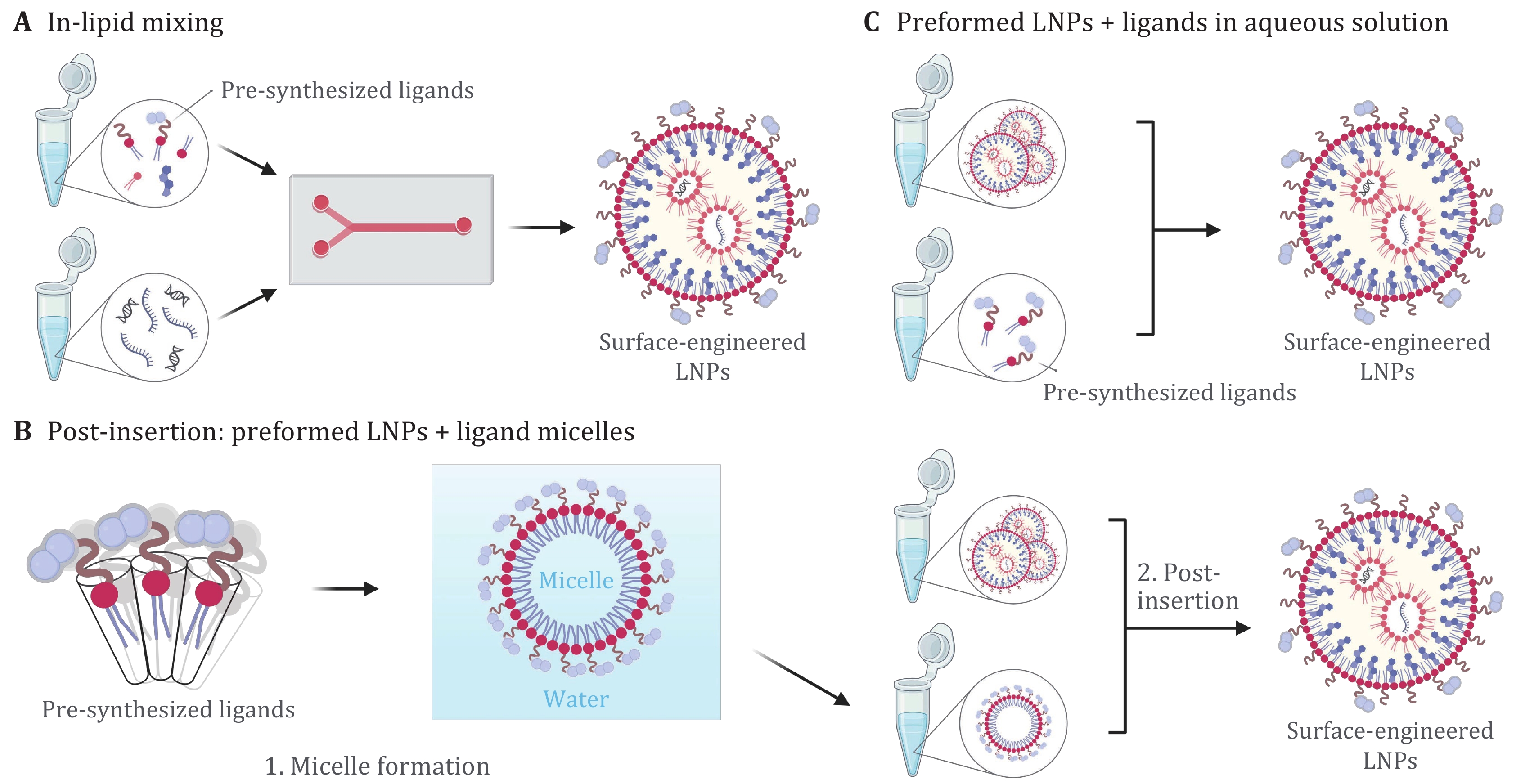
Figure 2. Incorporation methods of pre-synthesized ligands for generating surface-engineered LNPs. A Pre-synthesized ligands are dissolved in ethanol with lipid mixtures, and surface-engineered LNPs are prepared by the direct “in-lipid mixing” method. B Micelles containing pre-synthesized ligands and LNPs are first formed separately, and surface-engineered LNPs are prepared by the post-insertion method. C Pre-synthesized ligands are dissolved in aqueous solution, and surface-engineered LNPs are formed by incubation of preformed LNPs with the ligands. Created with BioRender.com
-
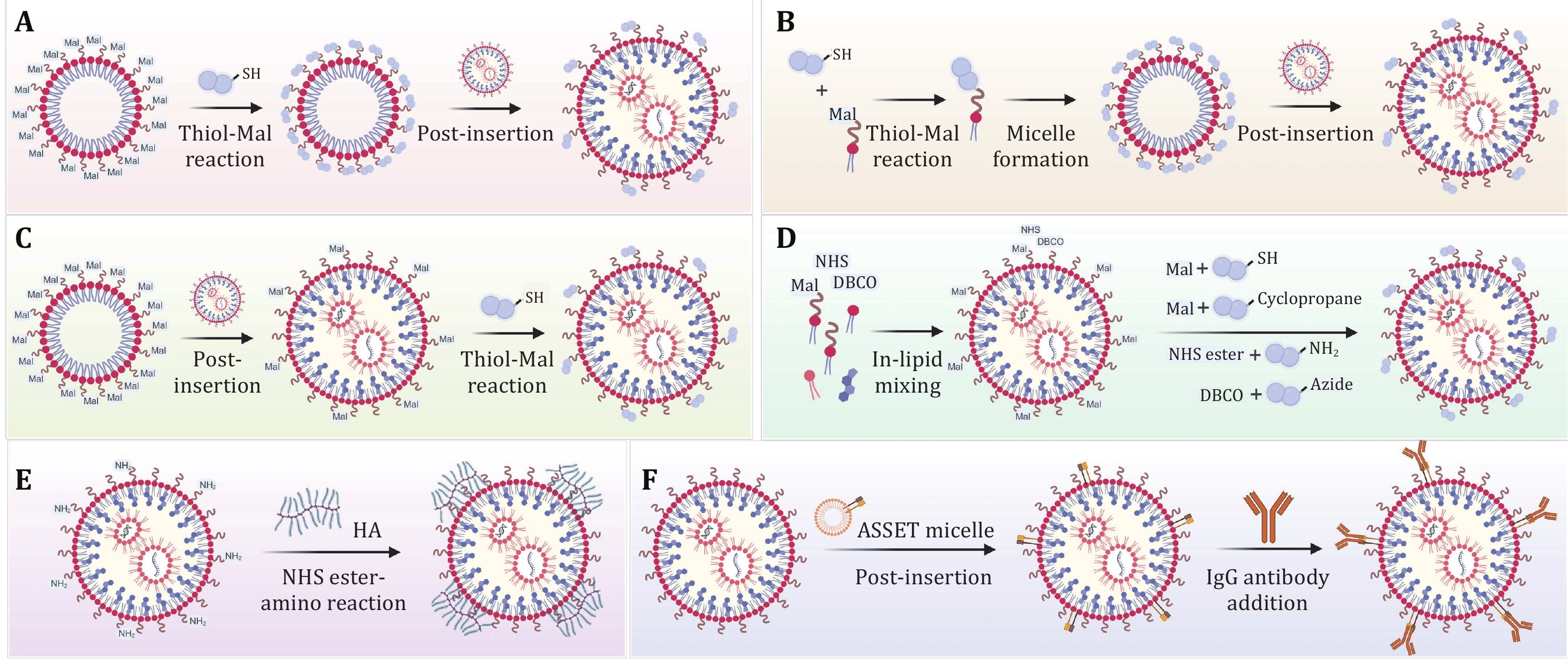
Figure 3. In situ modification strategies for generating surface-engineered LNPs. A Ligand-micelles are first formed by incubation of preformed Mal-micelles with ligands containing thiol groups, and surface-engineered LNPs are prepared by the post insertion method. B Ligand-PEG-lipids are first synthesized by Thiol-Mal reaction, and then ligand-micelles are formed; surface-engineered LNPs are finally prepared by the post insertion method. C Mal-LNPs are first formed from Mal-micelles by the post-insertion method, and surface-engineered LNPs are prepared through Thiol-Mal reaction. D Functionalized LNPs are formed by the “in-lipid mixing” method, and surface-engineered LNPs are prepared through different reactions. E HA-LNPs are prepared through NHS ester-amino reaction. F ASSET molecules are first inserted into LNPs, and surface-engineered LNPs are prepared following the addition of IgG antibodies. Created with BioRender.com
-
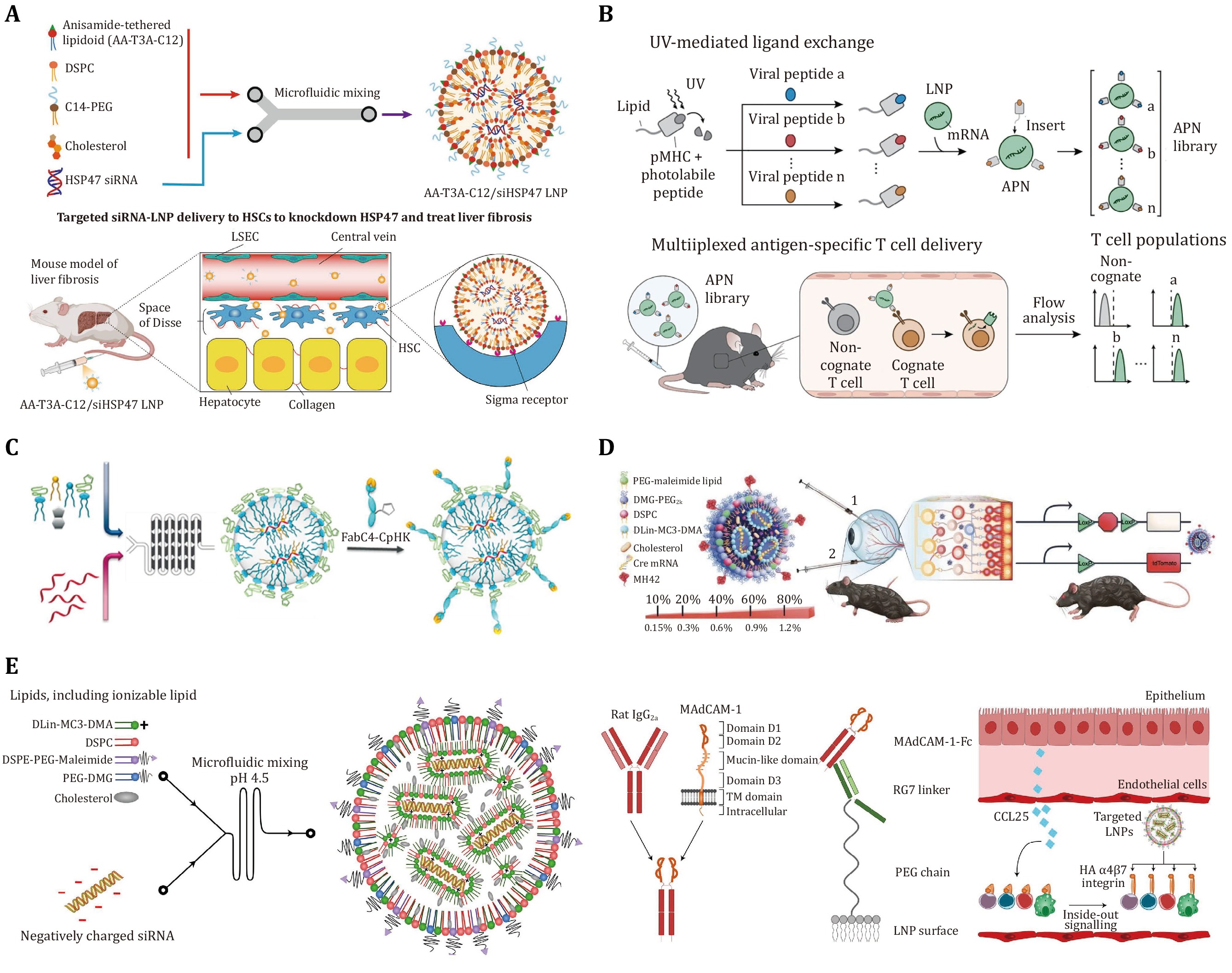
Figure 4. Surface-engineered LNPs for targeted nucleic acid delivery. A Formulation of AA-T3A-C12/siHSP47 LNP via microfluidic mixing and schematic illustration of targeted delivery to activated HSCs to knockdown HSP47 and treat liver fibrosis. Adapted from Han et al. (2023) with permission. B Schematic illustration of UV-mediated peptide exchange of MHCI APNs for in vivo multiplexed delivery to virus-specific T cells. Adapted from Su et al. (2022) with permission. C Schematic illustration of αPV1-LNPs construction via microfluidic mixing. Adapted from Li et al. (2020) with permission. D Schematic illustration of LNP formulation and conjugation with peptide via maleimide-thiol chemistry and Cre mouse model depicting both routes of administration trialed. Adapted from Herrera-Barrera et al. (2023) with permission. E Generation of LNPs to target the high-affinity conformation of integrin α4β7. Adapted from Dammes et al. (2021) with permission
-
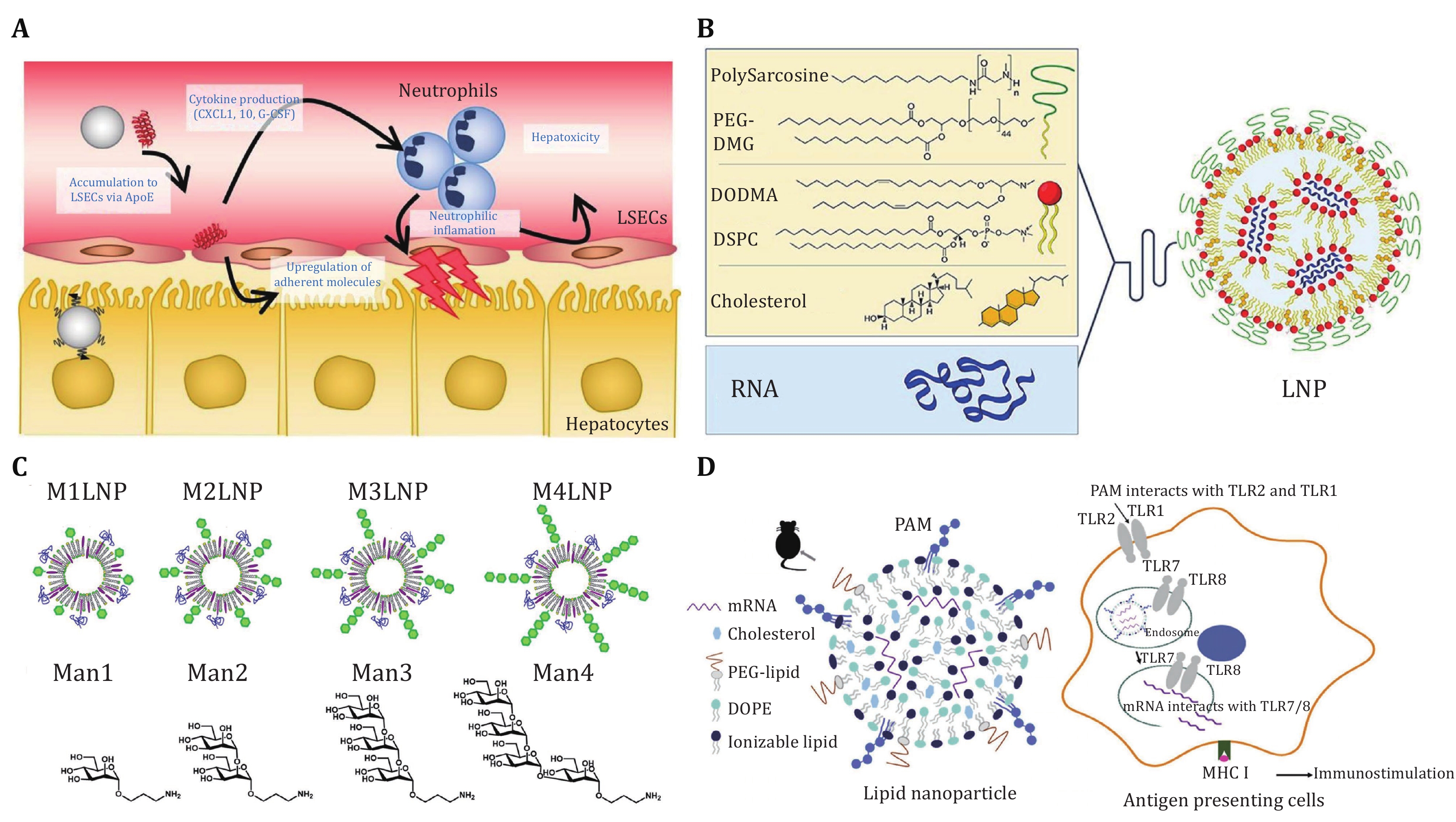
Figure 5. Surface-engineered LNPs for other purposes beyond targeted delivery. A Schematic illustration of highly specific delivery of siRNA to hepatocytes, which circumvents endothelial cell-mediated lipid nanoparticle-associated toxicity. Adapted from Sato et al. (2017) with permission. B Lipid composition used for pSar-LNP manufacturing. Adapted from Nogueira et al. (2020) with permission. C LNPs modified with mono-(M1), di-(M2), tri-(M3), and tetramannoside (M4) and chemical structure of mannose ligands Man1 (M1), Man2 (M2), Man3 (M3), and Man4 (M4) connected to linker. Adapted from Goswami et al. (2021) with permission. D Schematic illustration of the Pam3 incorporated LNPs encapsulating mRNA vaccines (Pam-LNPs) for the efficient immune stimulation and subsequent mRNA-mediated cancer immunotherapy. Adapted from Lee et al. (2020) with permission
Figure
5 ,Table
0 个