-
近年来,新精神活性物质(NPS)在世界范围内流行,其成瘾性强、易合成且难以管控,造成了严重的社会危害[1]。目前,全球已出现近1 000种NPS,主要包括合成大麻素、合成卡西酮、苯乙胺、色胺和苯环己哌啶等,其中,合成卡西酮类多达140种,常被不法分子以“浴盐”“清洗剂”“植物肥料”等名义售卖。滥用合成卡西酮会产生幻觉、幻听等精神病症状,严重时还会伴有失眠、食欲不振、抑郁、焦虑、自残等副作用[2-4],甚至会攻击他人[5]。但因其生产工艺简单、原料易得,且多通过网上销售,使得合成卡西酮的监管和打击难度极大,现已成为近年来国际禁毒领域最突出的难题之一。
合成卡西酮主要是不法分子通过对卡西酮或甲卡西酮进行细微的结构修饰获得的,因此存在许多结构类似物。4-氟甲卡西酮(4-FMC)是甲卡西酮的氟取代衍生物,是继第一代合成卡西酮4-甲卡西酮之后的第二代合成卡西酮类物质[6],于1952年首次合成,2015年被列管。3-氟甲卡西酮(3-FMC)是4-FMC的位置异构体,其结构示于图1。尽管两者化学性质相似,能产生相似的兴奋和致幻效果[7],但包括我国在内的很多国家尚未对3-FMC进行列管。因此,准确鉴定3-FMC和4-FMC对毒品犯罪案件中毒品鉴定和吸毒认定具有重要意义。
合成卡西酮的结构分析主要采用液相色谱-质谱(LC-MS)、气相色谱-质谱(GC-MS)、红外光谱(IR)、核磁共振波谱(NMR)等方法。Archer等[8]开发了一种使用FTIR和19F NMR快速测定氟甲基卡氮二酮位置异构体的方法。然而,IR和NMR因灵敏度不足,不适用于复杂混合物分析[9]。色谱-质谱技术具有质量范围宽、分辨率高、检测精度高等特点,适用于复杂混合物中目标物质的快速筛查,因而被广泛应用于新型毒品的结构鉴定[10-12]。Liu等[13]采用液相色谱-飞行时间质谱和气相色谱-静电场轨道阱高分辨质谱,系统研究了3种新合成卡西酮类物质的质谱裂解途径。
本文采用液相色谱-四极杆-静电场轨道阱高分辨质谱(LC-Q-Orbitrap/MS)和气相色谱-四极杆飞行时间质谱(GC-QTOF MS),对氟甲卡西酮位置异构体3-FMC和4-FMC在电子电离质谱(EI-MS)和电喷雾电离-碰撞诱导解离串联质谱(ESI-CID-MS/MS)模式下产生的主要碎片离子和裂解过程进行分析,并结合密度泛函理论计算方法研究其裂解机理,以更准确地阐明异构体的质谱行为和结构特征,为法庭科学实验室鉴定合成卡西酮提供参考。
-
GC-QTOF MS气相色谱-质谱联用仪:美国安捷伦公司产品,配有自动进样器;Dionex Ultimate 3000液相色谱仪、Thermo Q Extractive四极杆-静电场轨道阱高分辨质谱仪:美国赛默飞公司产品。
-
甲醇、乙腈:HPLC级,德国默克公司产品;甲酸:HPLC级,ROE Scientific Inc公司产品;3-FMC(CAS号:1049677-77-1;分子式:C10H12FNO;分子质量:181.21)和4-FMC(CAS号:447-40-5;分子式:C10H12FNO;分子质量:181.21)标准品:上海原思标物科技有限公司产品;水:由Millipore Milli-Q-Gradient净化系统制得。
-
用甲醇分别溶解3-FMC和4-FMC对照品。GC-QTOF MS、LC-Q-Orbitrap/MS分析浓度分别为1 mg/L、100 μg/L。
-
色谱条件:HP-5MS毛细管柱(30 m×250 μm,0.25 μm);进气温度300 ℃;柱流速1.0 mL/min;分流比40:1;载气为氦气(纯度>99.9%);升温程序为初始温度50 ℃,保持3 min,以20 ℃/min升至320 ℃,保持13 min。
质谱条件:EI电离源(70 eV),质量扫描范围m/z 25~200,离子源温度230 ℃,四极杆温度150 ℃。
-
色谱条件:Hypersil GOLD VANQUISH色谱柱(100 mm×2.1 mm, 1.9 μm);流动相为乙腈(A)和1%甲酸水溶液(B);梯度洗脱条件为0~2 min(95%B),2~9.5 min(95%~5%B),9.5~12 min(5%B);流动相流速0.3 mL/min;进样量10 μL。
质谱条件:加热电喷雾电离源正离子模式(HESI+);质量扫描范围m/z 160~600;碰撞气体为氮气;喷淋电压3 800 V;毛细管温度350 ℃;传动毛细管温度350 ℃;全扫描数据采集方式:full MS-ddMS2;质量分辨率14 000,自动增益控制(AGC)目标为3×106,最大注射时间(IT)50 ms;质量分辨率35 000,AGC目标1×105,最大IT 50 ms;隔离窗为m/z 4。
-
使用Gaussian16程序,在(U)M06-2X-D3/def2-SVP理论[14-16]水平下对各稳定结构和过渡态进行几何优化和频率计算。使用ORCA(5.0.2版本)程序,在RI-DLPNO-CCSD(T)/cc-pVTZ理论能级[17-19]和相应的辅助基组[20]下获得单点能量,并在对应质谱条件的温度和101.325 kPa下计算吉布斯自由能的热力学校正量。
-
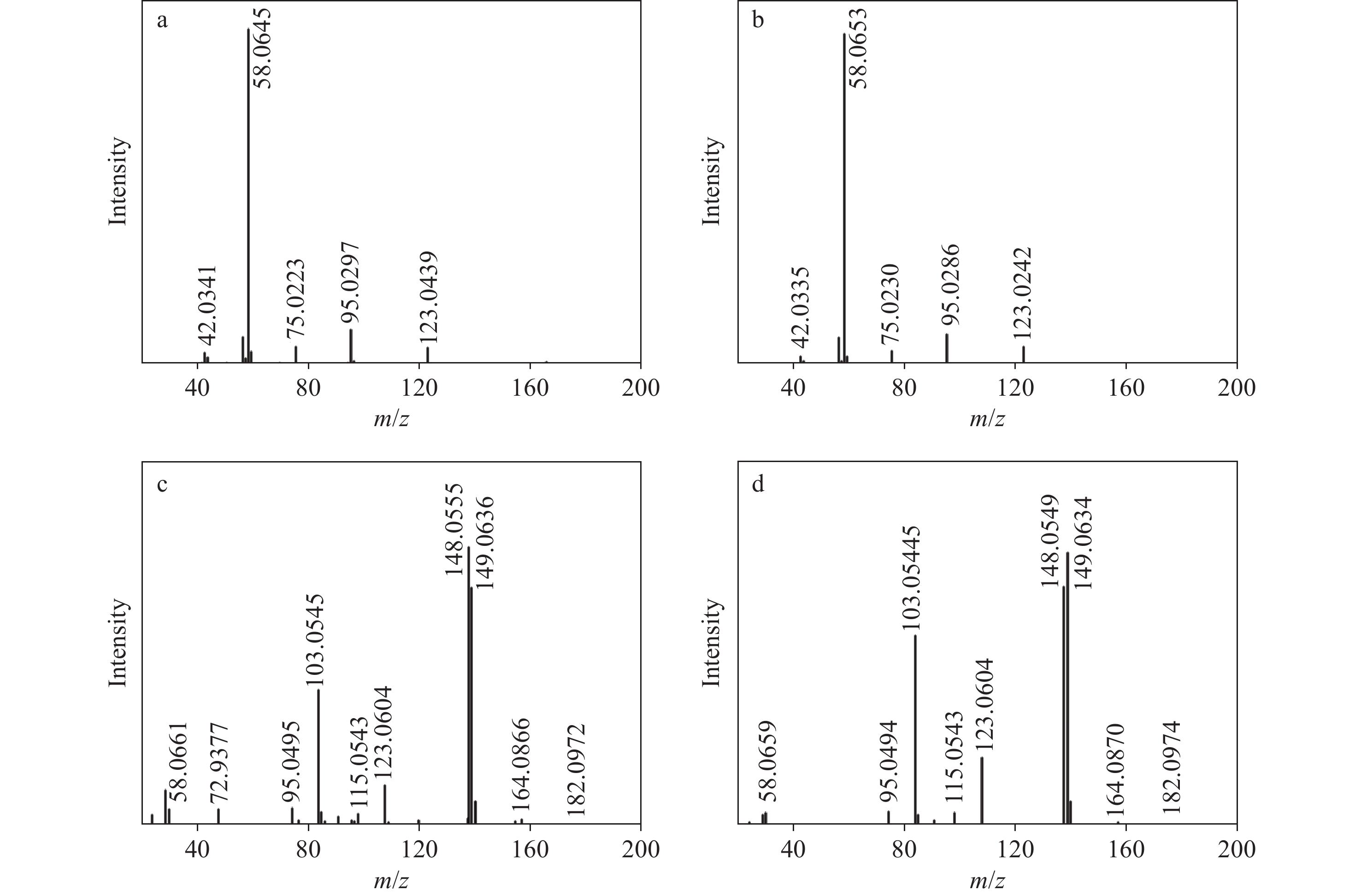
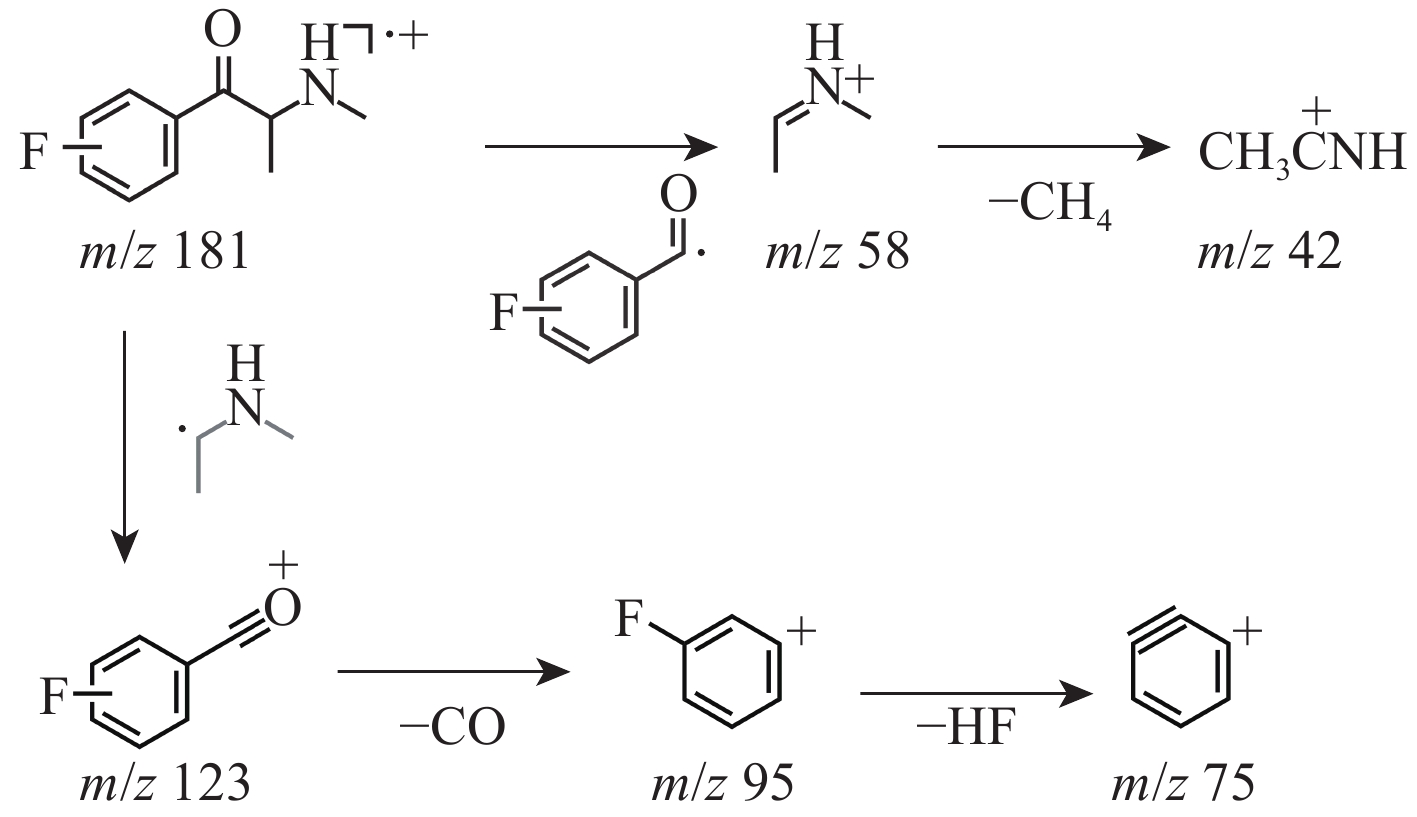
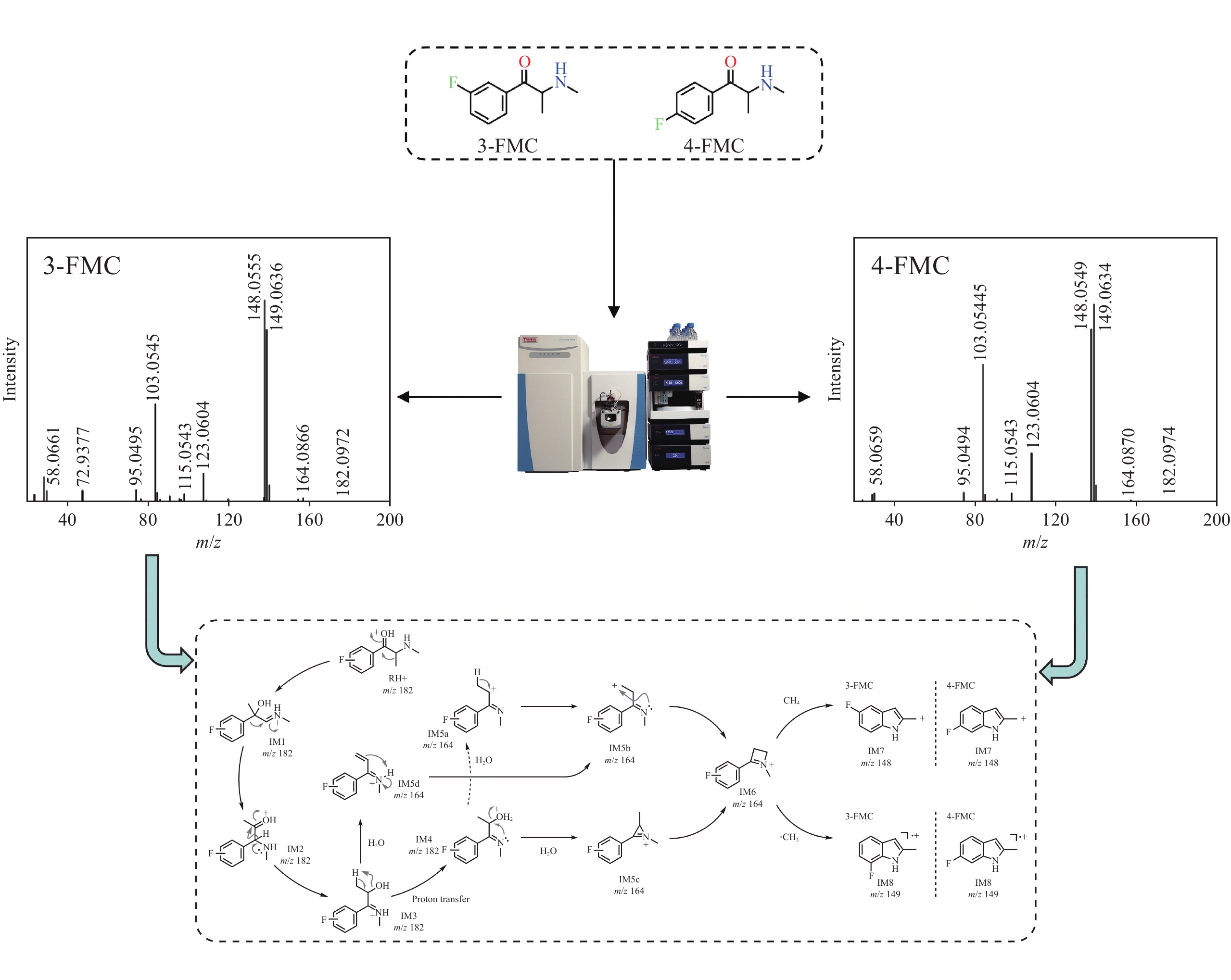
3-FMC和4-FMC的气相色谱峰的EI-MS质谱图分别示于图2a、2b,裂解途径示于图3,两者具有相似的碎片离子和裂解方式。前体离子m/z 181经化学键断裂生成碎片离子m/z 123和58。碎片离子m/z 123失去1分子CO生成碎片离子m/z 95,进一步失去1分子HF生成碎片离子m/z 75。另一方面,碎片离子m/z 58失去1分子CH4生成碎片离子m/z 42。
-
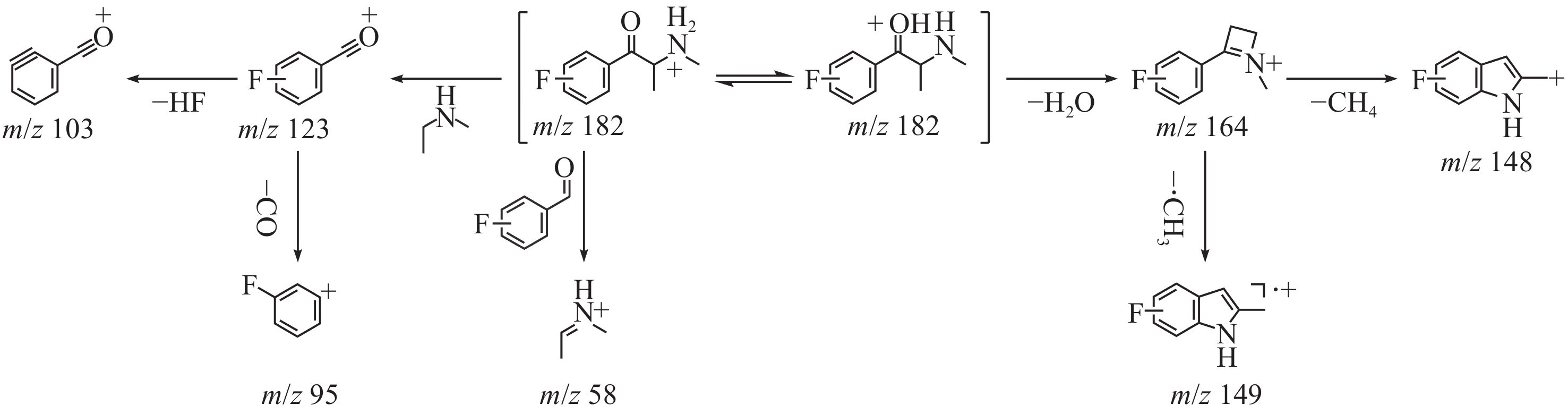
3-FMC和4-FMC的液相色谱峰的ESI-CID-MS/MS二级质谱图分别示于图2c、2d。与EI-MS不同,在ESI-CID-MS/MS模式下,虽然3-FMC与4-FMC具有相似的碎片离子,但丰度明显不同。3-FMC和4-FMC的ESI-CID-MS/MS碎裂途径示于图4。母离子m/z 182丢失1分子H2O生成碎片离子m/z 164,进一步丢失1分子CH4生成碎片离子m/z 148,或失去1分子甲基自由基生成碎片离子m/z 149。母离子m/z 182还可通过苯环侧链碳键断裂生成碎片离子m/z 123和58。此外,碎片离子m/z 123丢失1分子HF或1分子CO后,分别生成碎片离子m/z 103或95。
-
对比图2a和2b可发现,3-FMC和4-FMC的碎片离子及其丰度基本相似,这是因为EI属于硬电离方式,且卡西酮类物质的分子离子稳定性较低,在高能电子冲击下,3-FMC和4-FMC的分子离子无法稳定存在,易发生碎裂。此外,分子离子峰的缺失可能会给合成卡西酮异构体的鉴定带来困难。而ESI属于软电离方式,可为异构体鉴定提供更多的结构信息。氟原子具有吸电子诱导效应和给电子共轭效应,其位于苯环的邻位或对位,对共轭体系的稳定性具有显著影响。共轭体系越稳定,自由基电荷越分散,自由基稳定性越高,越有利于自由基生成。因此,当氟原子在4位发生取代时,一方面,其与苯环、苯环对位侧链上的双键和氨基形成大共轭体系,有助于分散自由基电荷;另一方面,氟原子的强吸电子诱导效应会使苯环对位取代基上的电子云密度降低,进一步分散自由基电荷,更有利于自由基稳定,从而有助于甲胺发生均裂并失去1分子甲基自由基,形成碎片离子m/z 149。当氟原子在3位发生取代时,虽然共轭体系仍存在,但与4位取代相比,氟原子的吸电子诱导效应无法有效降低间位取代基的电子云密度,分散自由基电荷的能力减弱,易发生异裂并丢失1分子CH4生成碎片离子m/z 148。
-
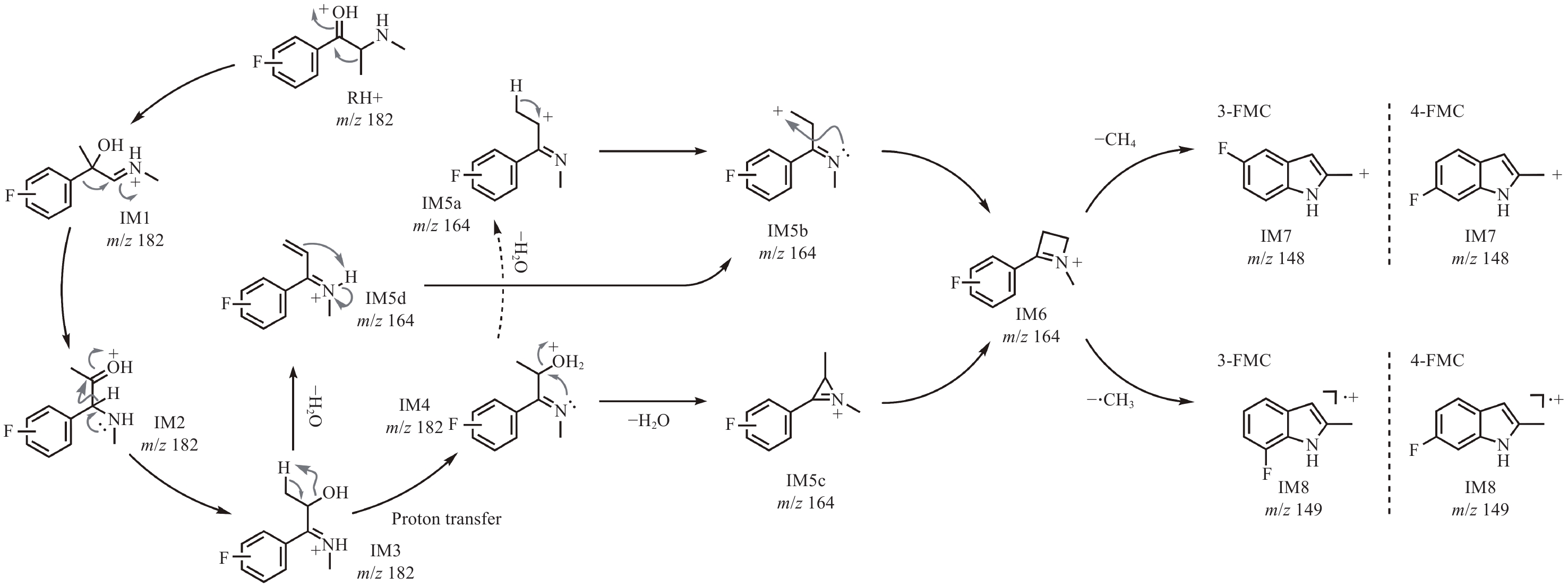
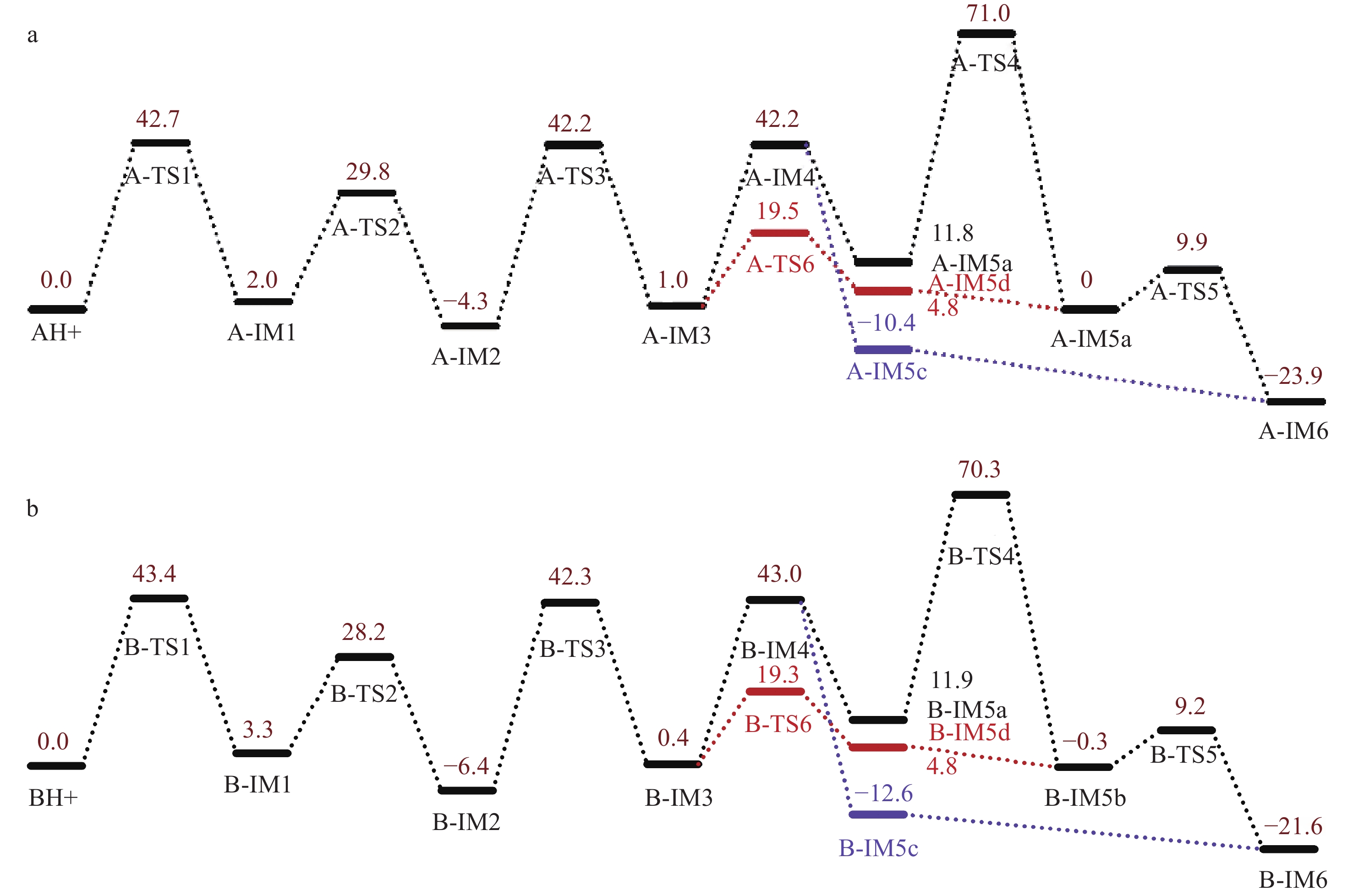
为进一步分析3-FMC和4-FMC的ESI-CID-MS/MS谱图差异,采用计算化学法研究二者的裂解机理。结果表明,3-FMC和4-FMC的ESI-CID-MS/MS裂解过程被中间体IM5系列(IM5a、IM5b、IM5c和IM5d)和IM6分为2个阶段,示于图5。第一阶段为分子内脱水成环反应。首先通过三步迁移生成N-质子化的β-羟基亚胺异构体(IM3),IM3为脱水反应提供了合适的结构基础,这也是α-羟基N-苯基胺重排反应[21]的一种变体。中间体IM5经系列异构化形成具有四元环侧链的稳定中间体IM6,这是碎片离子m/z 164产生的主要原因。由于IM3-TS6-IM5d的能垒较低,热力学上更具优势,该阶段的反应主要沿IM3-IM5d-IM5b-IM6路径进行,通过β-消除脱水、质子转移和分子内成环三步完成。3-FMC和4-FMC生成中间体IM6的吉布斯自由能分布情况示于图6。可以看出,由3-FMC和4-FMC生成中间体IM6具有相似的裂解机理。由中间体IM3生成IM6的最有利途径为中间体IM5d直接脱水,3-FMC和4-FMC完成该过程分别需要克服77.4和79.1 kJ/mol的能垒。由于裂解过程中没有外部质子源,经IM4(172.5和178.2 kJ/mol)途径的分子内质子转移在能量上是不利的。此外,在动力学上,更易发生从IM5b到IM6的环合反应,其能垒更低,分别为41.4和39.5 kJ/mol。
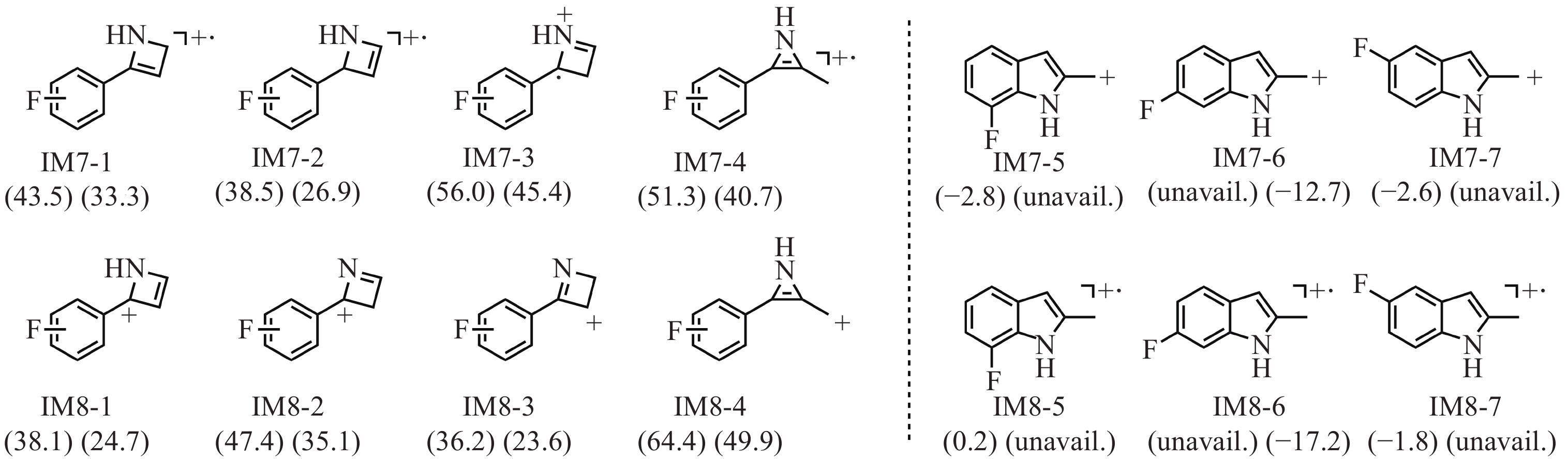
基于一般经验无法推断中间体IM6进一步裂解生成碎片离子IM7(m/z 148)和IM8(m/z 149)的过程。因此,需通过相对吉布斯自由能进行理论预测,对所有保留氟苯基结构的可能异构体进行筛选,才能确定这2个未知片段的最稳定结构。基于相对吉布斯自由能筛选出的IM7和IM8可能结构示于图7。可见,IM7-5和IM8-7是3-FMC生成相应碎片的最有利结构,对于4-FMC来说,则是IM7-6和IM8-6,且这些结构所需的能量在电喷雾离子化二级质谱裂解的合理能量范围之内。对于3-FMC,IM7-5(−11.7 kJ/mol)的相对吉布斯自由能比IM8-5(0.8 kJ/mol)低;而对于4-FMC,IM8-6(−72.0 kJ/mol)的相对吉布斯自由能比IM7-6(−53.1 kJ/mol)低。ESI-CID-MS/MS结果表明,3-FMC的碎片离子m/z 148的丰度高于碎片离子m/z 149,而4-FMC则相反,这与理论分析一致。
-
本工作采用GC-QTOF MS和LC-Q-Orbitrap/MS研究氟甲卡西酮位置异构体3-FMC和4-FMC的质谱裂解模式。GC-QTOF MS结果表明,3-FMC和4-FMC具有相似的碎片离子和离子丰度。LC-Q-Orbitrap/MS结果表明,尽管3-FMC和4-FMC具有相似的碎片离子,但碎片离子m/z 148和149的丰度明显不同。这是因为氟原子具有较强的吸电子诱导效应,可以显著降低苯环的电子云密度,尤其是苯环邻、对位的电子云密度。因此,苯环侧链上的甲氨基会发生不同的裂解反应,导致碎片离子m/z 148和149的丰度不同。密度泛函理论研究进一步证实了该实验结果,并根据吉布斯自由能谱和电子性质推导出详细的裂解途径:第一步裂解(失去1分子H2O)是α-羟基N-苯基亚胺重排反应的变体,由3步α-迁移构成,形成四元环侧链碎片离子(m/z 164, IM6),此过程为决速步骤;第二步通过相对吉布斯自由能进行理论预测,对所有保留氟苯基结构的可能异构体进行筛选,确定最稳定结构。综上,LC-Q-Orbitrap/MS法可准确有效地鉴别氟甲卡西酮位置异构体3-FMC和4-FMC,为合成卡西酮的鉴定和管控提供依据。
氟甲卡西酮异构体的质谱识别和计算化学研究
Mass Spectrometry Identification and Computational Chemistry Study of Fluoromethcathinone Isomers
-
摘要: 合成卡西酮是一类新精神活性物质,具有成瘾性强、种类多、更新快、结构相似等特点。3-氟甲卡西酮(3-FMC)和4-氟甲卡西酮(4-FMC)是第二代合成卡西酮类物质氟甲卡西酮的2种位置异构体,其结构非常相近,对二者的准确定性分析是当前司法鉴定中的一个难题。本文采用气相色谱-四极杆-飞行时间串联质谱(GC-QTOF MS)和高效液相色谱-四极杆-轨道阱串联质谱(LC-Q-Orbitrap/MS)技术,结合计算化学方法研究位置异构体3-FMC和4-FMC的质谱裂解模式和裂解机理。结果表明,3-FMC的苯环侧链易断裂并产生碎片离子m/z 58。由于氟原子具有较强的吸电子诱导效应,使苯环邻位和对位的电子云密度发生显著变化,导致不同位置异构体的苯环侧链发生了不同的裂解方式。其中,3-FMC苯环上侧链易发生异裂失去1分子CH4生成碎片离子m/z 148,而4-FMC苯环上侧链易发生均裂并失去1分子CH3·生成碎片离子m/z 149。进一步利用密度泛函理论,根据相对吉布斯自由能筛选裂解可能生成片段的结构,确认最稳定的异构体碎片离子。质谱分析实验结果与计算化学结论一致,本研究为准确鉴定氟甲卡西酮异构体3-FMC和4-FMC提供了参考依据。Abstract: Synthetic cathinones are a class of new psychoactive substances, characterized by strong addictiveness, great diversity, rapid updates, and similar structures. Among them, 3-fluoromethcathinone (3-FMC) and 4-fluoromethcathinone (4-FMC) are two positional isomers of the second-generation synthetic cathinone, fluoromethcathinone. Their structures are very similar, therefore, the accurate qualitative analysis of these isomers remains a challenge in current judicial appraisals. In this study, the fragmentation patterns of positional isomers 3-FMC and 4-FMC were investigated by gas chromatography-quadrupole time-of-flight mass spectrometry (GC-QTOF MS) and liquid chromatography-Q-Orbitrap mass spectrometry (LC-Q-Orbitrap/MS). Then, the mass differences of 3-FMC and 4-FMC fragments were further analyzed by the relationship between chemical structures and fragment ion abundances. Computational studies further demonstrated the experimental results using Gibbs free energy profiles and electronic properties of possible fragments. In addition, the fragmentation mechanisms of 3-FMC and 4-FMC were investigated by applying density functional theory calculation, which can more accurately elucidate the mass spectral behavior and structure characteristics of compounds as reflected by mass spectrometric data. The results showed that the side chain of the benzene ring in 3-FMC is prone to break and generate a fragment ion at m/z 58. It was found that the electron cloud densities at the ortho and para positions of the benzene ring change significantly due to the strong electron-withdrawing inductive effect of the fluorine atom. This, in turn, leads to different fragmentation patterns of the benzene ring side chains of different positional isomers. Specifically, the side chain on the benzene ring of 3-FMC is prone to heterolytic cleavage to lose one molecule of CH4 and generate a fragment ion with m/z 148. In contrast, the side chain on the benzene ring of 4-FMC tends to homolytic cleavage to lose one molecule of CH3· and generate a fragment ion at m/z 149. Structures of possible fragments were screened based on relative Gibbs free energy to confirm the most stable isomer of fragment ions. The first stage of fragmentation with one H2O loss is a variant of α-hydroxy N-phenylimines rearrangement reaction, which consists of three steps of σ-migration and acts as the rate-determinant step of the fragmentation process to form the four-membered ring side chain fragment (m/z 164, IM6). Further theoretical prediction was conducted using relative Gibbs free energy. All possible isomers that retain the fluorophenyl structure are screened to determine the most stable structure. The results of the fragmentation patterns obtained from computational chemistry are consistent with the experimental results of mass spectrometry analysis. The study provides an important reference and a basis for the accurate identification of the fluoromethcathinone isomers 3-FMC and 4-FMC.
-

-
-
[1] JANKOVICS P, VÁRADI A, TÖLGYESI L, LOHNER S, NÉMETH-PALOTÁS J, KŐSZEGI-SZALAI H. Identification and characterization of the new designer drug 4’-methylethcathinone (4-MEC) and elaboration of a novel liquid chromatography-tandem mass spectrometry (LC-MS/MS) screening method for seven different methcathinone analogs[J]. Forensic Science International, 2011, 210(1/2/3): 213 -220 .[2] ROJEK S, KŁYS M, MACIÓW-GŁĄB M, KULA K, STRONA M. Cathinones derivatives-related deaths as exemplified by two fatal cases involving methcathinone with 4-methylmethcathinone and 4-methylethcathinone[J]. Drug Testing and Analysis, 2014, 6(7/8): 770 -777 .[3] GATCH M B, DOLAN S B, FORSTER M J. Methylenedioxymethamphetamine-like discriminative stimulus effects of seven cathinones in rats[J]. Behavioural Pharmacology, 2020, 31(4): 378 -384 . doi: 10.1097/FBP.0000000000000540[4] DARGAN P I, SEDEFOV R, GALLEGOS A, WOOD D M. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone)[J]. Drug Testing and Analysis, 2011, 3(7/8): 454 -463 .[5] MARUSICH J A, GRANT K R, BLOUGH B E, WILEY J L. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice[J]. NeuroToxicology, 2012, 33(5): 1 305-1 313. [6] GATCH M B, RUTLEDGE M A, FORSTER M J. Discriminative and locomotor effects of five synthetic cathinones in rats and mice[J]. Psychopharmacology, 2015, 232(7): 1 197-1 205. [7] MEYER M R, VOLLMAR C, SCHWANINGER A E, WOLF E, MAURER H H. New cathinone-derived designer drugs 3-bromomethcathinone and 3-fluoromethcathinone: studies on their metabolism in rat urine and human liver microsomes using GC-MS and LC-high-resolution MS and their detectability in urine[J]. Journal of Mass Spectrometry, 2012, 47(2): 253 -262 . doi: 10.1002/jms.2960[8] ARCHER R P. Fluoromethcathinone, a new substance of abuse[J]. Forensic Science International, 2009, 185(1/2/3): 10 -20 .[9] BONETTI J. Mass spectral differentiation of positional isomers using multivariate statistics[J]. Forensic Chemistry, 2018, 9: 50 -61 . doi: 10.1016/j.forc.2018.06.001[10] 范一雷, 陈显鑫, 吴剑丙, 张宏建, 吴昊, 徐雨. 新型苯二氮卓类策划药氯地西泮异构体质谱识别[J]. 分析试验室, 2023, 42(7): 891 -896 . FAN Yilei, CHEN Xianxin, WU Jianbing, ZHANG Hongjian, WU Hao, XU Yu. Identification of new designer benzodiazepine chlorodiazepam isomers by mass spectrometry[J]. Chinese Journal of Analysis Laboratory, 2023, 42(7):891 -896 (in Chinese).[11] 范一雷, 陈显鑫, 张宏建, 吴昊, 徐雨. 质谱识别新型合成苯环己哌啶类物质氟胺酮异构体[J]. 分析试验室, 2023, 42(3): 338 -343 . FAN Yilei, CHEN Xianxin, ZHANG Hongjian, WU Hao, XU Yu. Differentiation of novel synthetic phenylcyclohexyl piperidines fluamine isomers by mass spectrometry[J]. Chinese Journal of Analysis Laboratory, 2023, 42(3):338 -343 (in Chinese).[12] 范一雷, 陈显鑫, 薛锦锋, 吴昊, 柯星, 徐雨. 新型靛红腙类合成大麻素质谱裂解规律研究[J]. 分析试验室, 2024, 43(1): 57 -63 . FAN Yilei, CHEN Xianxin, XUE Jinfeng, WU Hao, KE Xing, XU Yu. Investigation of mass spectrometry-based fragmentation patterns of new “OXIZID” synthetic cannabinoids[J]. Chinese Journal of Analysis Laboratory, 2024, 43(1):57 -63 (in Chinese).[13] LIU C, HUA Z, SONG C, JIA W. Identification and analytical characterization of N-propyl norbutylone, N-butyl norbutylone, N-benzyl norheptedrone, and N-pyrrolidinyl-3,4-DMA[J]. Drug Testing and Analysis, 2023, 15(1): 47 -57 . doi: 10.1002/dta.3358[14] VUJOVIĆ M, RAGAVENDRAN V, ARSIĆ B, KOSTIĆ E, MLADENOVIĆ M. DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones[J]. Open Chemistry, 2020, 18(1): 185 -195 . doi: 10.1515/chem-2020-0021[15] GRIMME S, HANSEN A, BRANDENBURG J G, BANNWARTH C. Dispersion-corrected mean-field electronic structure methods[J]. Chemical Reviews, 2016, 116(9): 5 105-5 154. [16] WANG Y, VERMA P, JIN X, TRUHLAR D G, HE X. Revised M06 density functional for main-group and transition-metal chemistry[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(41): 10 257-10 262. [17] DUTTA A K, SAITOW M, DEMOULIN B, NEESE F, IZSÁK R. A domain-based local pair natural orbital implementation of the equation of motion coupled cluster method for electron attached states[J]. The Journal of Chemical Physics, 2019, 150(16): 164 123. [18] HERNÁNDEZ VERA M, JAGAU T C. Resolution-of-the-identity approximation for complex-scaled basis functions[J]. The Journal of Chemical Physics, 2019, 151(11): 111 101. [19] PAPAJAK E, LEVERENTZ H R, ZHENG J, TRUHLAR D G. Efficient diffuse basis sets: cc-pVxZ+ and maug-cc-pVxZ[J]. Journal of Chemical Theory and Computation, 2009, 5(12): 3 330. [20] BERNHOLDT D E, HARRISON R J. Fitting basis sets for the RI-MP2 approximate second-order many-body perturbation theory method[J]. The Journal of Chemical Physics, 1998, 109(5): 1 593-1 600. [21] SEKUŁA K, ZUBA D. Structural elucidation and identification of a new derivative of phenethylamine using quadrupole time-of-flight mass spectrometry[J]. Rapid Communications in Mass Spectrometry, 2013, 27(18): 2 081-2 090. -


 首页
首页 登录
登录 注册
注册



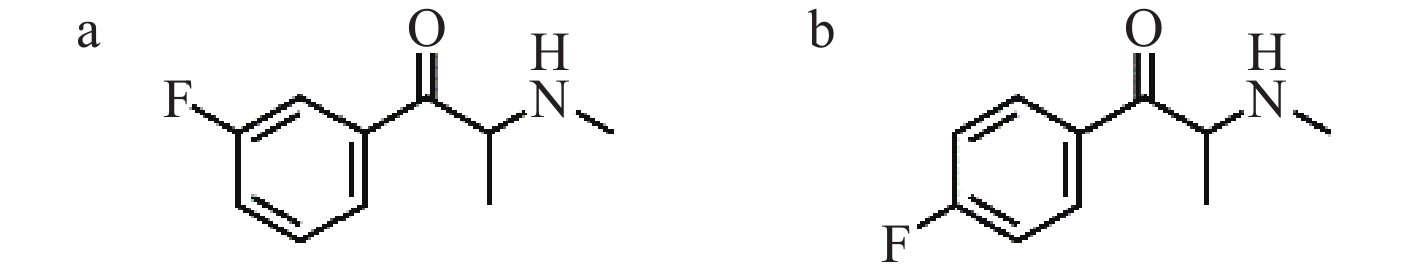
 下载:
下载: