-
过渡金属(transition metals,TMs)主要指元素周期表中第ⅢB~Ⅷ族的元素,独特的未填满d轨道的电子结构使其具有优异的功能特性。过渡金属碳化物(transition metal carbides,TMCs)主要指第ⅣB~Ⅷ族元素(如Hf、Ta、W)与碳元素(C)形成的化合物,由于过渡金属与碳元素之间的强共价键及金属键特性,过渡金属碳化物具有高熔点、高硬度、抗腐蚀和优异的导电性及力学性能,被广泛应用于磨削刀具、涂层、核燃料防护以及超高声速飞行器热防护系统(thermal protection system, TPS)[1–2]中。
在制备过渡金属碳化物的众多方法中,传统方法如化学气相沉积法(chemical vapor deposition,CVD)、自蔓延高温合成法(self-propagation high temperature synthesis, SHS)、机械合金法(mechanical alloying, MA)等已具备成熟的工艺[3–4],但随着科学技术的不断进步和工业需求的迅速提升,人们对过渡金属碳化物性能的要求日益严苛,传统制备技术的局限性也逐渐显露,例如,材料纯度受限、晶格结构难以精准控制以及工艺耗时长等。因此,研究人员尝试了更多制备高性能过渡金属碳化物的途径,如放电等离子烧结(spark plasma sintering, SPS)、高频感应加热烧结(high frequency induction-heated sintering, HFIHS)、高温高压(high pressure and high temperature, HPHT)烧结等。其中,高温高压烧结是指在高温和极端高压条件下处理反应物,通过调控该过程中的温度和压力参数,诱导反应物产生特殊的相变与晶体重构,使超高压条件下制备的过渡金属碳化物具有常压条件下难以实现的高密度相结构,提升材料的致密度、纯度和结构稳定性,使其在极端环境应用中表现出显著的优势。此外,超高压环境能够促进碳化物晶体的形成,在一定程度上减小晶粒尺寸,并提高其均匀性,使过渡金属碳化物的电学、机械和热学性能表现更加优异。
本文拟简述数种过渡金属碳化物(主要集中于ⅣB~ⅥB族)制备方法,并从制备方法、力学性能以及微观机制3方面入手,梳理近年来几种典型过渡金属碳化物的研究进展,特别关注高温高压烧结方法在过渡金属碳化物制备中的应用,希望通过对现有研究的全面回顾,为未来高性能过渡金属碳化物陶瓷的制备和应用提供一定的启发。
-
过渡金属碳化物粉体和涂层的制备方法多种多样:直接碳化法[5]采用过渡金属粉末与碳进行反应,反应过程难以控制,可能生成多相化合物,需要对反应物进行球磨后再进行化学提纯;化学气相沉积法[6]利用气相化合物或单质在气相界面上进行化学反应沉积,从而得到反应物薄膜;自蔓延高温合成法[7]通过物质的放热反应传导,在极短的时间内形成化合物;机械合金法[8]通过球磨转动产生冲击和碰撞作用,使其中的材料发生结构、性能改变或化学反应,从而得到反应物粉体;微波法[9]利用材料介质损耗将微波能转变为热能进行烧结。上述工艺已经相对成熟,然而,对于过渡金属碳化物的实际工业应用而言,制备高致密、性能优异的块体陶瓷至关重要。
-
过渡金属碳化物陶瓷主要采用碳化物粉体或金属单质+碳粉为初始原料烧结制备。过渡金属碳化物陶瓷块体烧结方法包括常见的直接高温烧结、热压烧结(hot press sintering, HPS)[10–12]以及更先进的烧结技术,如放电等离子烧结[13–15]、高频感应加热烧结[16–18]。然而,由于过渡金属碳化物的金属与碳元素间的强共价键和碳原子的低扩散系数,使得采用上述方法制备陶瓷块体需要极高的温度,从而导致晶粒粗化,并且难以实现更高的致密度[19]。迄今为止,诸多研究工作一直致力于改善烧结方法和工艺参数。一种途径是通过加入烧结添加剂,降低烧结温度或增加陶瓷密度[20–21],然而添加剂虽然可以降低烧结条件和提高材料致密化,但也引入了新的物相,增加了结构的复杂性,并限制了对材料本征性质的理解。另一种途径则是高温高压烧结。在高压下,材料的体积减小,共价键键长缩短,内部能态发生变化,有效降低了陶瓷固结的活化能。更重要的是,高压可以抑制晶粒生长,并且避免了烧结添加剂引入的二次相。因此,高温高压烧结具有降低烧结温度、缩短烧结时间、抑制晶粒生长、提高产物的致密化程度[22–23]、物相纯净等优点。高温高压烧结可分为预压成型、高压组装配置、升压、通电加热、卸温卸压5个步骤。Feng等[24]和Silvestroni等[10]分别对碳化铪(hafnium carbide, HfC)粉体进行放电等离子烧结(在2 300 ℃、100 MPa条件下保持30 min)和热压烧结(在1 900 ℃、30 MPa条件下保持20 min),得到了相对密度大于96%和89%的块体陶瓷材料。Liang等[25]和He等[26]利用高温高压方法,在1 700 ℃、15 GPa 条件下仅烧结10 min,得到的陶瓷的相对密度分别达到了99.2%和98.7%。这说明高温高压烧结方法对于合成高致密度的过渡金属碳化物陶瓷具有重要意义。
-
近年来,高超声速飞行器和火箭推进器的蓬勃发展促使人们不断开发新型材料,以适应高负载、超高温、强侵蚀的环境。超高温陶瓷(ultra-high temperature ceramics, UHTCs)因具有极高的熔点(高于3 000 ℃)、高硬度、高导热性和导电性以及在高温下的稳定性,有望成为新一代高温防护材料[1, 27–28]。目前,UHTCs主要包括过渡金属碳化物、硼化物和氮化物。而作为过渡金属碳化物中熔点最高(高于3 900 ℃)的陶瓷材料[29–30],HfC被用于火箭推进、核反应堆、火箭喷嘴、航空航天和热场发射器材料。因此,如何制备性能优异的HfC陶瓷块体是迫切需要解决的问题。
Sciti等[31]将HfC粉末在2 200 ℃、65 MPa条件下进行放电等离子烧结,获得了相对密度为98%、维氏硬度(Vickers hardness)为19 GPa的陶瓷块体;Cedillos-Barraza等[13]利用放电等离子烧结,在2 350 ℃、38 MPa 条件下合成了相对密度为85%、维氏硬度为10.2 GPa、断裂韧性(fracture toughness)为2.9 MPa·m1/2、杨氏模量仅为283 GPa的HfC块体;Silvestroni等[10]在1 900 ℃、30 MPa条件下,通过热压烧结获得了HfC陶瓷,其相对密度为89%,维氏硬度为5.79 GPa,断裂韧性为1.88 MPa·m1/2。
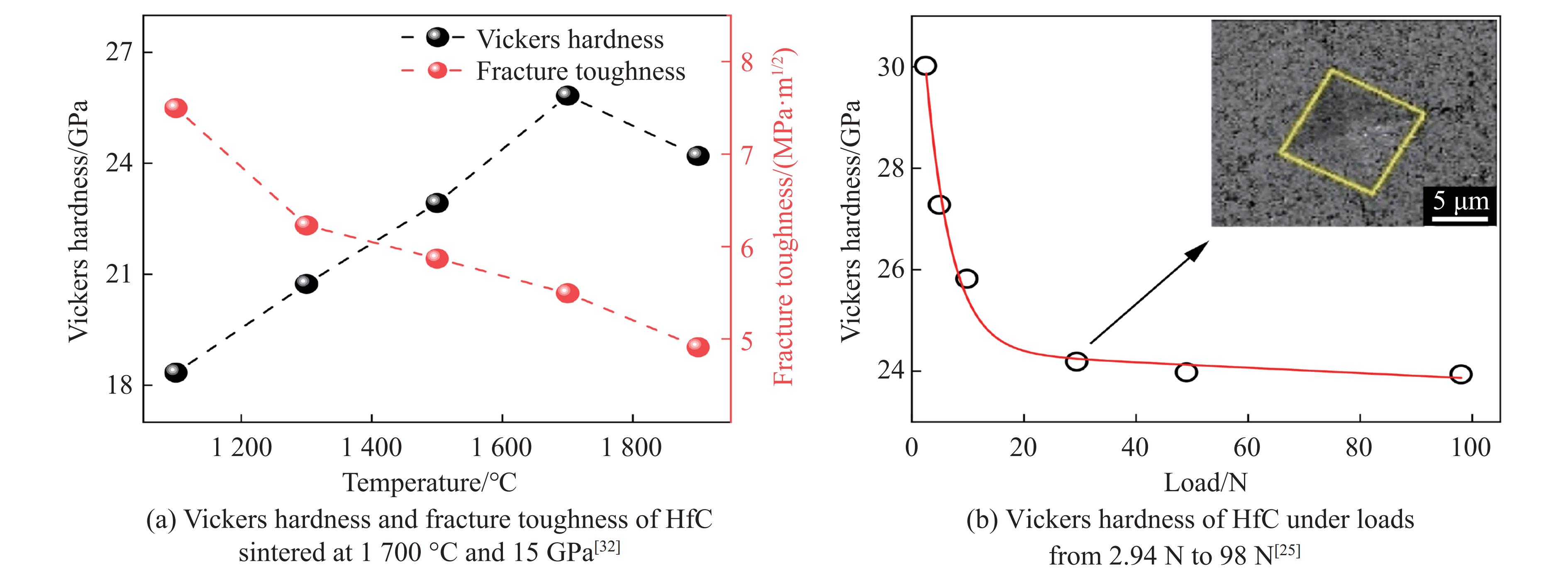
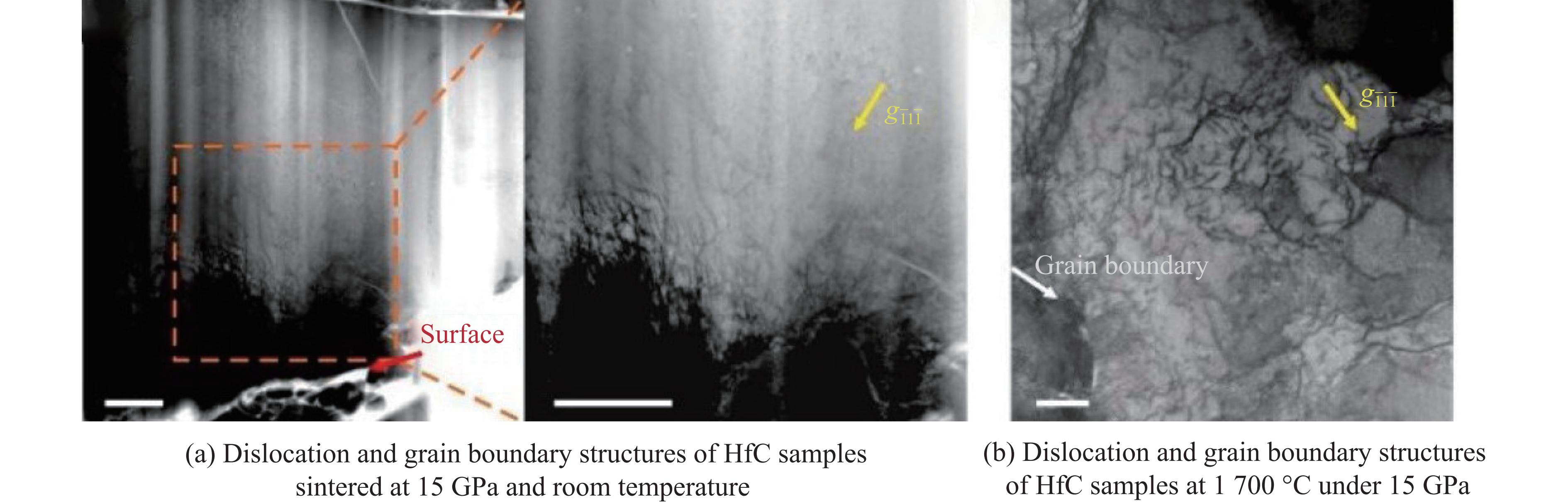
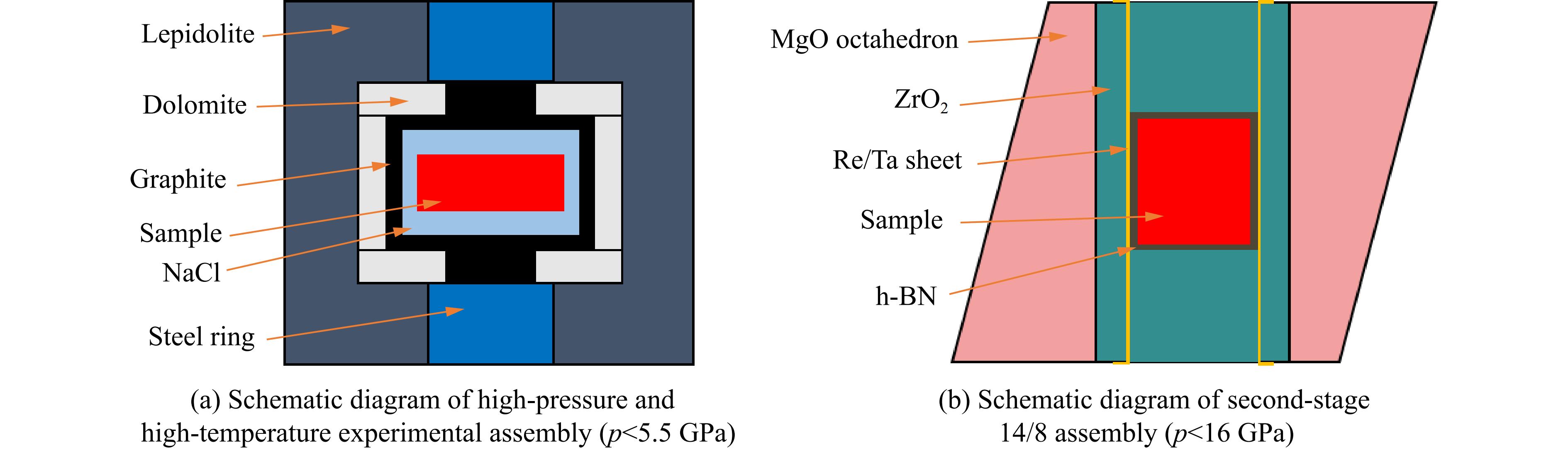
综上可知,熔点极高的HfC烧结成陶瓷所需温度高于2 000 ℃,而高温高压烧结方法使烧结难度降低,且产物的致密度更高、力学性能更优。Liang等[25]通过高温高压法将二氧化铪(HfO2)与石墨(C)在1 400 ℃、5.5 GPa条件下直接反应生成HfC,并将HfC在1 100~1 900 ℃、15 GPa条件下进行烧结[25, 32]。高温高压合成实验在大腔体六面顶压机上进行,2种压力范围的实验组装分别如图1(a)(一级组装,压力可达 5.5 GPa)和图1(b)(二级14/8组装,压力可达16 GPa)所示。力学性能与烧结温度的关系如图2(a)所示,其中断裂韧性随烧结温度的升高而单调减小,在1 700 ℃、15 GPa条件下得到最优异的致密度和力学性能,相对密度为99.2%~99.5%。测试不同载荷下的维氏硬度,如图2(b)所示,得到其收敛的维氏硬度为24.2 GPa,断裂韧性为5.0~5.5 MPa·m1/2,均远超热压烧结和放电等离子烧结的陶瓷样品。为了揭示压力对HfC陶瓷微观结构的影响,Liang等[32]利用明场扫描透射电子显微镜(scanning transmission electron microscope,STEM)成像对25 ℃、15 GPa下得到的样品进行了表征(图3(a))。结果表明,高密度位错局部地分布在晶粒表面附近,由于HfC颗粒的接触产生了局部高压区,导致其在高压下容易发生塑性形变。然而,在高温高压条件下,随着温度升高,剪应力快速下降,产生了更大的塑性形变。图3(b)显示了在1 700 ℃、15 GPa下烧结的HfC陶瓷的位错结构,这些位错均匀地分布在晶粒中,表明高温可以增强原子的长程扩散,增加高密度位错的迁移率。
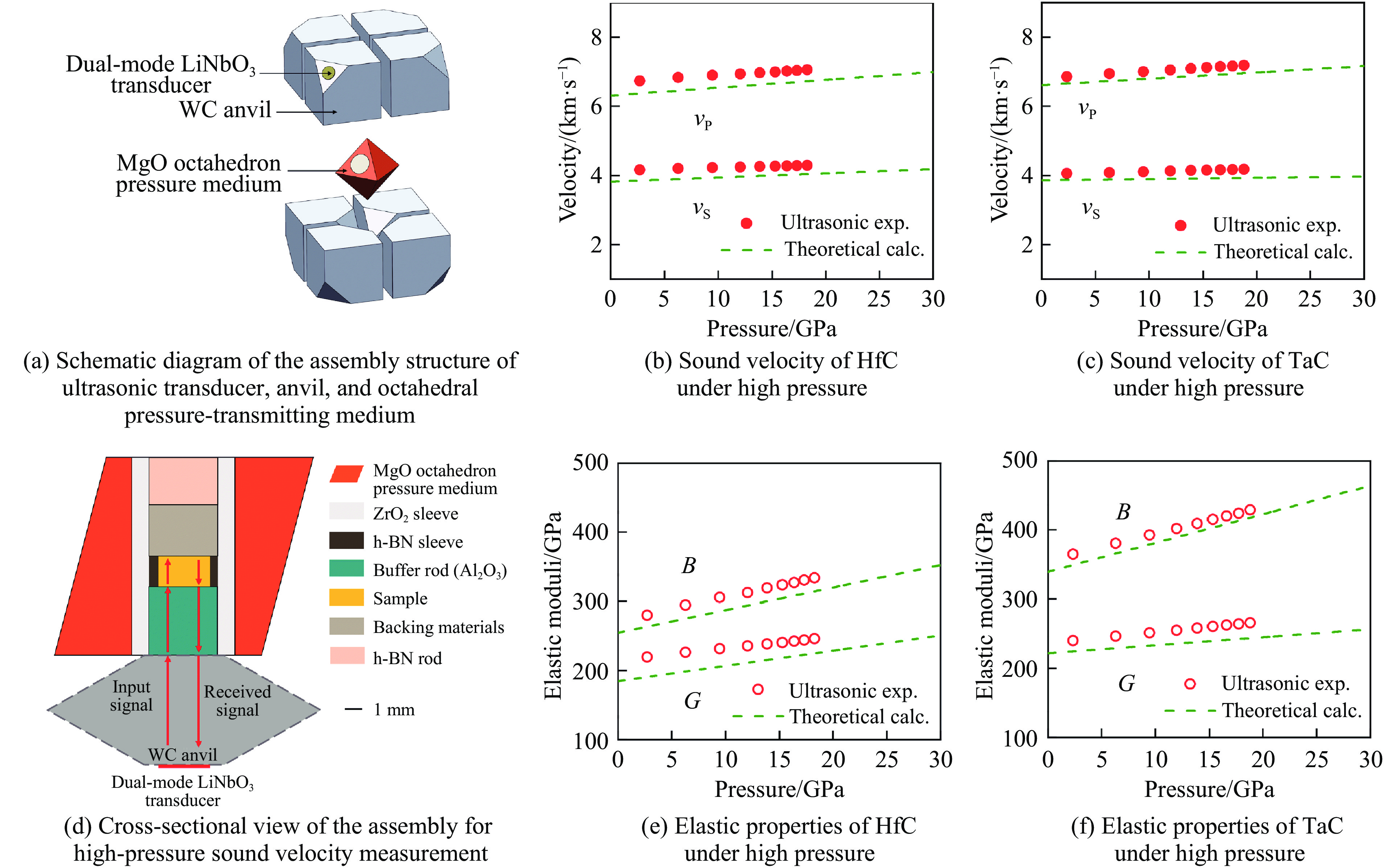
此外,He等[26]利用高压原位超声测量装置,研究了高温高压烧结合成的HfC和碳化钽(tantalum carbide, TaC)陶瓷在高压下的声速、弹性和力学性能。高压超声测量原理装置如图4(a)所示。将换能晶片LiNbO3(纵波P和横波S的中心频率分别为50和30 MHz)置于二级压砧碳化钨(tungsten carbide, WC)的截角处,由此发射出的声信号经二级压砧WC、缓冲棒Al2O3后到达样品,在声传播路径的每个介质变化的界面上会产生声回波,与发射波发生干涉(见如图4(d)),获取超声信号在样品中传播的时间[33],从而完成在高压下的超声测量。通过超声测量可以获得材料在高压下的弹性性质,包括体积模量(bulk modulus, B)、剪切模量(shear modulus, G)、杨氏模量(Young’s modulus, E)和泊松比(Poisson’s ratio, ν)。HfC在高压下的声速和弹性模量分别如图4(b)和图4(e)所示,可以看出,HfC陶瓷在高压下的纵波声速(vP)和横波声速(vS)、体积模量和剪切模量随压力升高几乎呈线性增长。此外,将现有声速和密度数据与有限应变方程[33]拟合,可以得到其在零压下的体积模量B0、剪切模量G0以及体积和剪切模量与压力p的依赖性。结果为:B0=272.6 GPa,G0=215.8 GPa,∂B/∂p=3.44,∂G/∂p=1.74,在零压下的杨氏模量E0=512.2 GPa,高于放电等离子烧结的结果(455 GPa)[31],说明高温高压烧结方法有利于过渡金属碳化物陶瓷展现出优异的力学性能。
-
碳化钛(titanium carbide, TiC)具有高熔点、高硬度,以及良好的抗氧化性、导电性和导热性,被广泛用作航空航天、高温电极、核反应堆和高速刀具材料[34–36]。因此,制备具有优异性能的碳化钛陶瓷块体是迫切需要解决的问题。关于TiC的制备,Ono等[37]在2 000 ℃、40 MPa条件下,通过热压烧结制备了相对密度为98%的TiC陶瓷。Teber等[38]和Abderrazak等[39]在1 650 ℃、100 MPa条件下,通过放电等离子烧结获得了相对密度分别为97.9%和95.1%、维氏硬度分别为25.7和27.0 GPa的TiC陶瓷块体。Shon等[16]在80 MPa压力下,通过球磨和高频感应加热烧结获得了维氏硬度为25.7 GPa的纳米TiC陶瓷。Fu等[40]在1 700 ℃条件下对亚微米TiC初始粉末进行无压烧结(pressureless sintering, PS),使其致密化至相对密度为95.7%、维氏硬度为20.3 GPa。
上述制备路线采用无压或低压烧结,难以获取更高相对密度、更优异性能的TiC陶瓷。Wang等[41]通过在1 500 ℃、9.0~14.0 GPa条件下烧结微米TiC粉末制备了多晶TiC陶瓷,并研究了其微观结构、密度、硬度和断裂韧性,揭示了相对密度、维氏硬度和断裂韧性对烧结压力的依赖性,如图5(a)和图5(b)所示。可以看出,其维氏硬度、断裂韧性和相对密度随着烧结压力的升高而增大。在1 500 ℃、14.0 GPa条件下烧结得到完全致密的TiC陶瓷,并且维氏硬度和断裂韧性分别达到31.2 GPa和4.2 MPa·m1/2。图5(c)给出了1 500 ℃、14.0 GPa下烧结样品的断裂面扫描电镜(scanning electron microscope, SEM)图像,可以观察到界面内存在大量的穿晶断裂,表现出优异的性能。高温高压环境导致晶粒产生严重的塑性变形,位错密度升高,从而提高了材料的硬度。
-
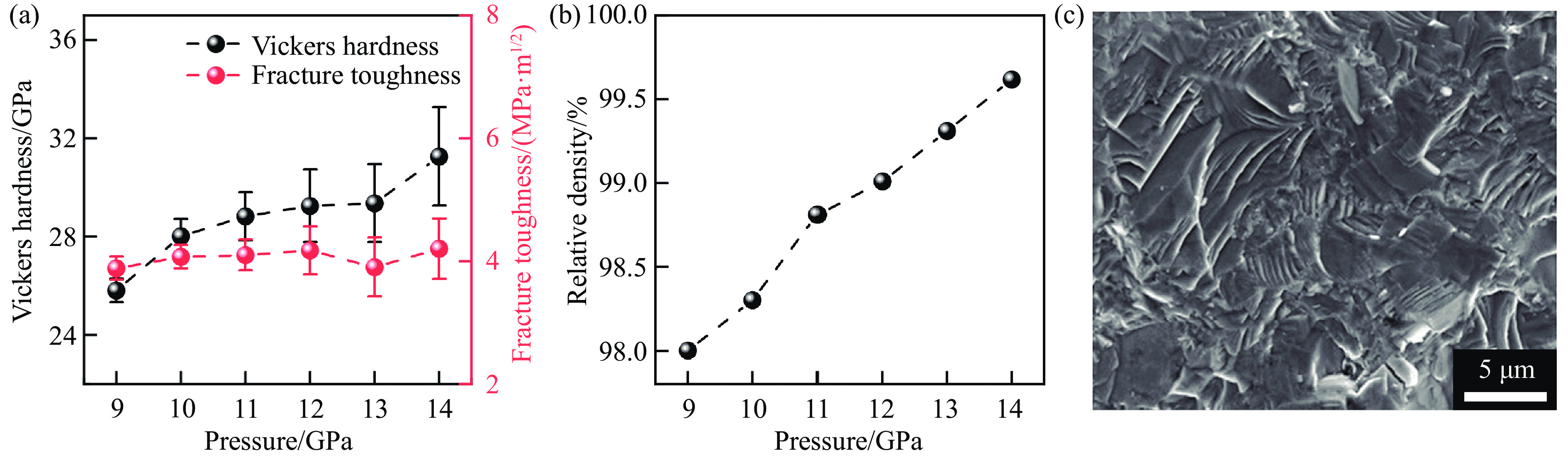
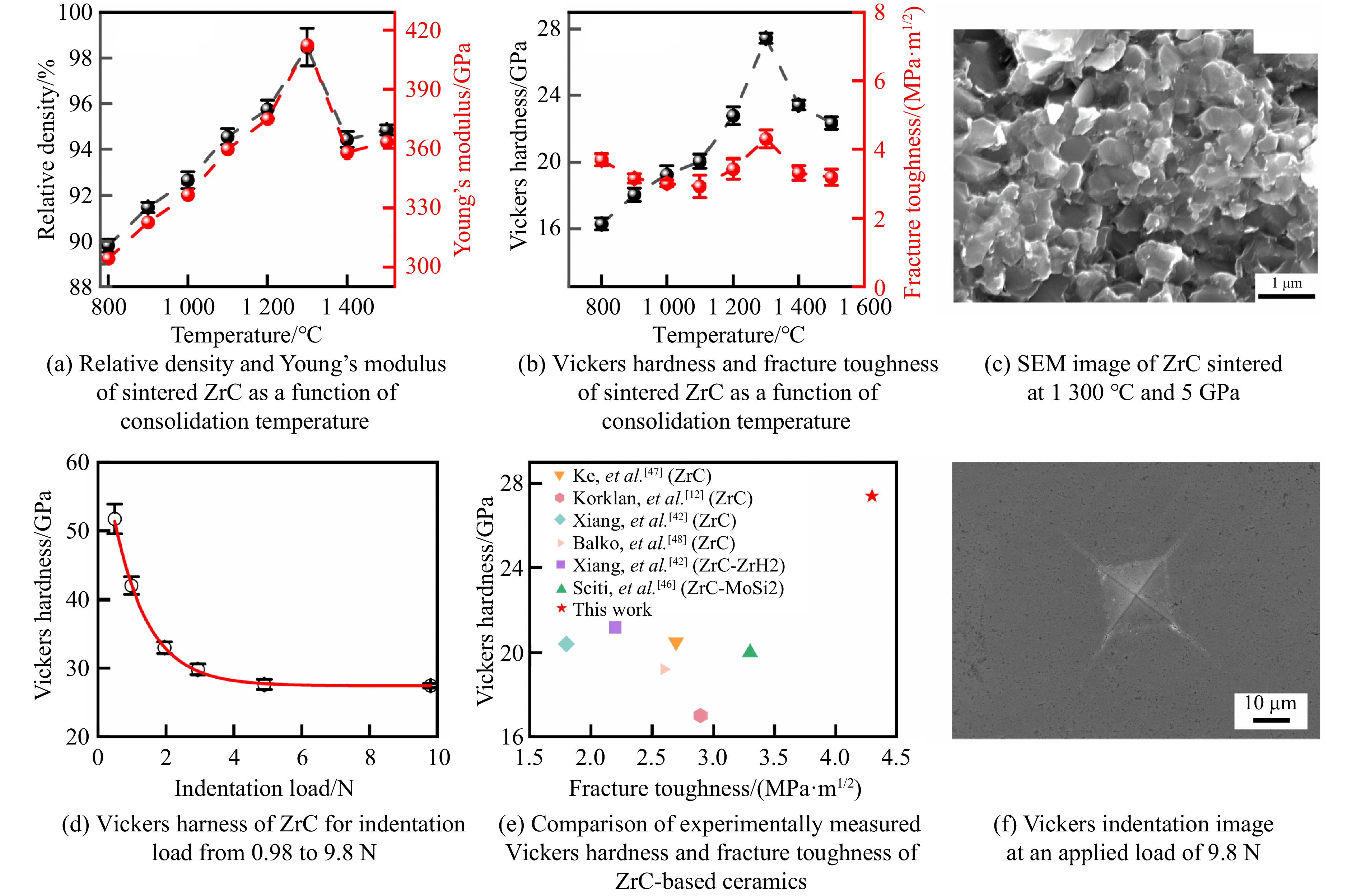
碳化锆(zirconium carbide, ZrC)因其密度低、熔点高、热稳定性优异以及中子透过性良好等特性,在极端环境下具有广阔的应用前景,被应用于核反应堆燃料包壳、高超声速飞行器热防护系统等。然而,ZrC陶瓷的制备面临挑战,传统方法通常需要极高的烧结温度和长时间保温以实现致密化,往往导致晶粒异常生长和力学性能下降。关于ZrC的制备工艺,Xiang等[42]采用等离子体辅助烧结(plasma-assisted sintering, PAS)技术,在1 600~1 850 ℃、100 MPa条件下制备了ZrC陶瓷,所得样品的维氏硬度为20.4 GPa,断裂韧性为1.8 MPa·m1/2。Acicbe等[43]采用放电等离子烧结技术,在1 750~1 850 ℃、40 MPa条件下制备了ZrC陶瓷,其维氏硬度为17.6 GPa,断裂韧性为3.3 MPa·m1/2。尽管这些方法在一定程度上实现了ZrC的致密化,但所需温度较高,且力学性能仍有提升空间。Yang等[44]通过高温高压方法,在800~1 500 ℃、5 GPa条件下成功制备了性能优异的纯相多晶ZrC陶瓷,并系统探究了烧结温度对ZrC微观结构和力学性能的调控机理。
在高温高压实验中,烧结温度显著影响ZrC陶瓷的致密化过程、微观结构和力学性能。在800 ℃时,由于晶界结合不够紧密,ZrC晶粒间存在大量孔隙,相对密度仅为89.8%,如图6(a)所示。随着烧结温度升高,孔隙率逐渐降低,致密度持续增加,在1 300 ℃时达到最致密状态,相对密度高达98.5%。此时,晶界结合显著增强,断裂模式由晶间断裂转变为穿晶断裂,如图6(c)所示。这种转变与高温高压下原子扩散受限、晶界滑移和重排增强密切相关。此时,其收敛的维氏硬度和断裂韧性分别为27.4 GPa和4.3 MPa·m1/2,如图6(b)和图6(d)所示,杨氏模量达到412 GPa。其中,杨氏模量由尼尔森模型[45]得到
式中:P和
$ \rho $ 分别为孔隙率和尼尔森形状因子。图6(f)为1 300 ℃、5 GPa条件下烧结的ZrC陶瓷在9.8 N载荷下维氏压痕的SEM图像。当温度高于1 300 ℃时,由于高温导致自由能过剩,ZrC陶瓷发生再结晶,导致晶粒异常生长,晶粒内部出现许多孔隙,致密度随之下降,与之相应的力学性能也同步下降。相比于传统烧结方法,高压条件能够降低烧结温度,从而在较低的温度下烧结出力学性能更优的ZrC陶瓷。图6(e)显示了在1 300 ℃、5 GPa条件下烧结的ZrC陶瓷与传统方法烧结[12, 42, 46–48]的ZrC陶瓷的维氏硬度和断裂韧性的对比,高温高压法制备的ZrC陶瓷硬度提高了29%~61%,韧性也优于先前报道的样品。这种性能提升的微观机制可归因于高温高压处理限制了原子的长程扩散,并且高压导致晶粒发生塑性变形,在晶粒内部引入大量微观缺陷(如孪晶和堆叠层错),阻碍位错在变形过程中的运动,从而导致加工硬化。此外,Yang等[44]还研究了高温高压烧结ZrC陶瓷的氧化行为。热重-差示扫描量热分析法(thermogravimetry-differential scanning calorimetry,TG-DSC)实验表明,在1 300 ℃、5 GPa下合成的样品的氧化起始温度为713 ℃,远高于传统方法制备样品的氧化起始温度200 ℃[49–50]。这表明,高温高压方法可同时优化ZrC陶瓷的力学性能以及热稳定性,使ZrC陶瓷在极端环境中的应用前景更加广阔。
-
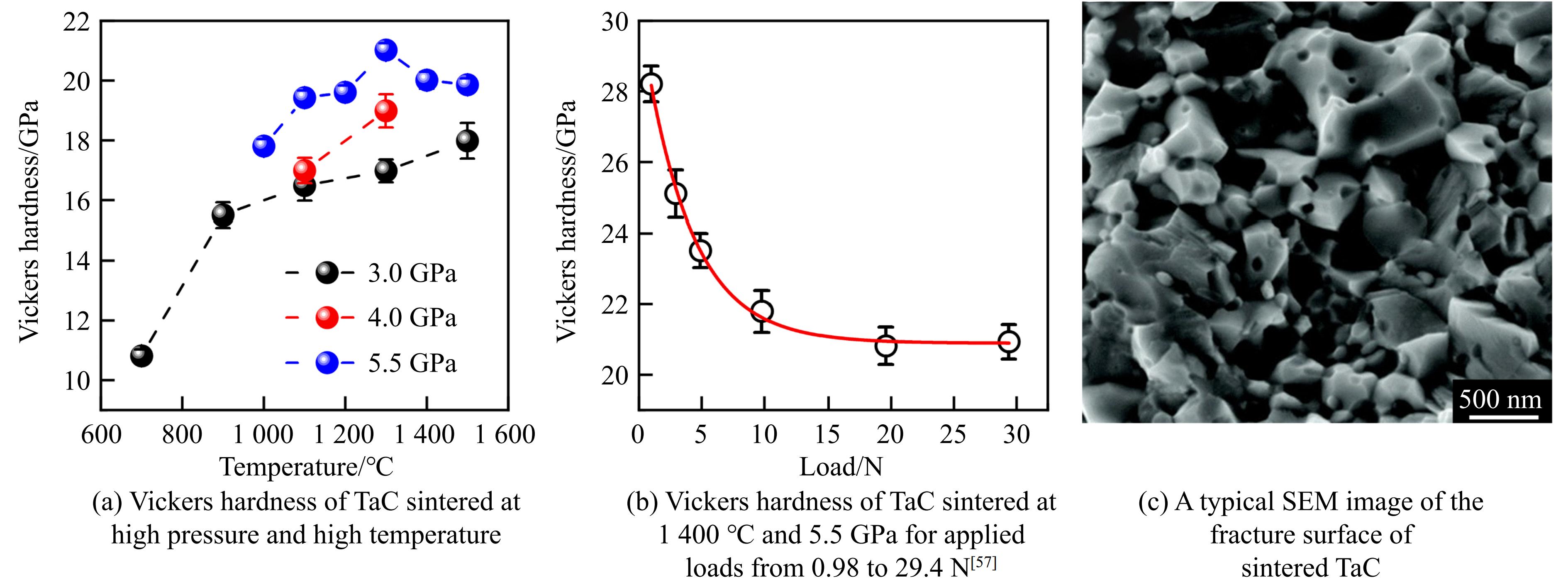
对于碳化钽(tantalum carbide, TaC)陶瓷,Zhang等[51–52]利用热压烧结技术,在2 400 ℃、30 MPa条件下合成的块体的相对密度为96%,且在2 300 ℃、30 MPa条件下合成了相对密度为94%、维氏硬度为14 GPa、断裂韧性为3 MPa·m1/2的纯相陶瓷;Cedillos-Barraza等[13]通过放电等离子烧结技术,在2 350 ℃、38 MPa条件下合成了相对密度为98%、维氏硬度为13.9 GPa、断裂韧性为2.7 MPa·m1/2、杨氏模量为458 GPa的TaC块体;Kim等[53]利用机械合金法与高频感应加热烧结方法,在80 MPa压力下得到的TaC陶瓷块体的维氏硬度为22 GPa、断裂韧性为5.1 MPa·m1/2;Rezaei等[54]在2 000 ℃、40 MPa条件下,采用热压烧结技术合成了相对密度为97%、维氏硬度为15.7 GPa、断裂韧性为4.1 MPa·m1/2的致密TaC陶瓷。
Chen等[55]和Zhang等[56]研究了高温高压烧结TaC陶瓷的力学性能,采用的压力范围为3.0~5.5 GPa,温度范围为700~1 500 ℃。如图7(a)所示,TaC陶瓷的维氏硬度与其烧结的压力呈正相关,在1 300 ℃、5.5 GPa时获得了最高的维氏硬度约21.0 GPa。Sun等[57]对在1 400℃、5.5 GPa条件下合成的样品进行了收敛维氏硬度测试及扫描电镜表征(图7(b)和图7(c)),获得的TaC陶瓷的收敛维氏硬度为20.9 GPa,扫描电镜图像显示,高温高压合成的陶瓷具有高致密性。
另外,He等[26]也对高温高压烧结的TaC陶瓷进行了高压超声测量(图4(c)和图4(f)),研究了其高压弹性,得到B0=355.9 GPa,G0=236.6 GPa,E0 =581.1 GPa,∂B/∂p=3.98,∂G/∂p=1.65。发现其弹性模量略高于Dodd等[58]的测量结果(B0=332 GPa,G0=234 GPa,E0=567 GPa),说明高压烧结有利于展现材料的本征力学性质。
-
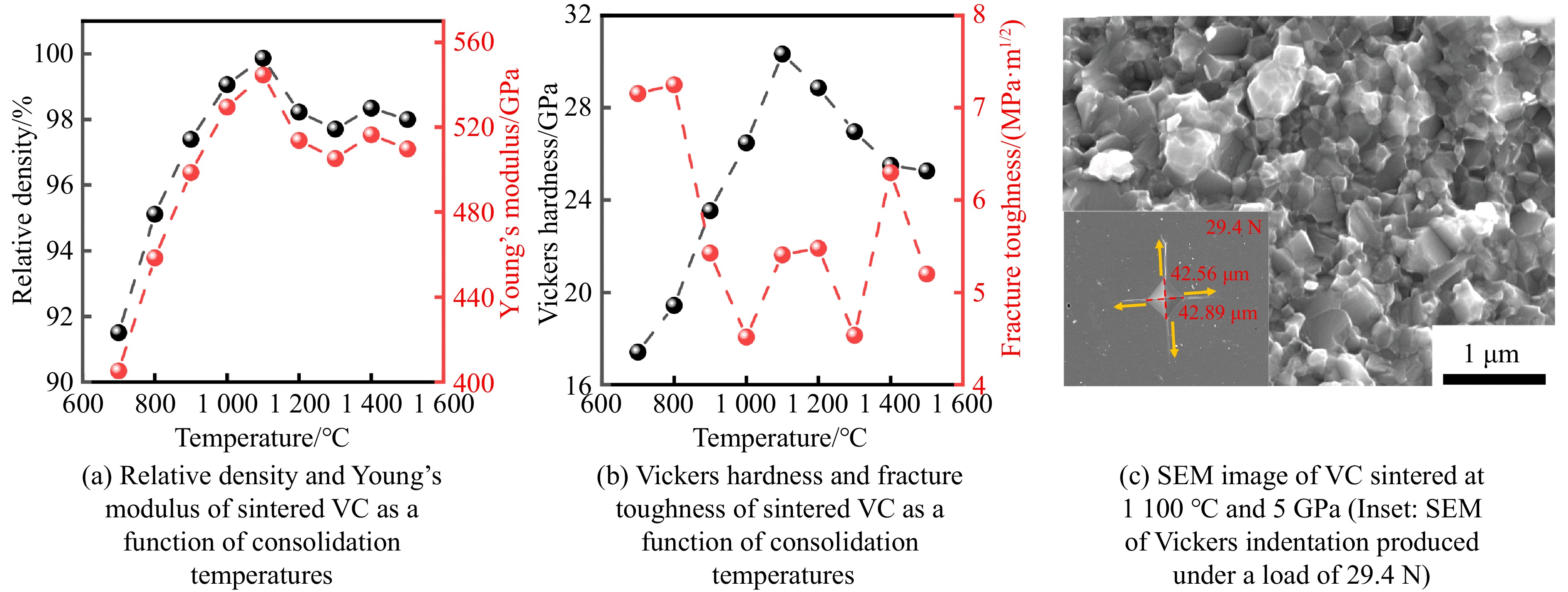
碳化钒(vanadium carbide, VC)陶瓷因硬度高、熔点高、耐磨性及化学稳定性优异,在超硬涂层、切削工具及极端环境防护材料领域展现出巨大的应用潜力。然而,由于V与C原子间存在强共价键结合及低扩散系数,导致采用传统烧结方法难以制备高致密块体陶瓷。针对这一挑战,Chen等[59]采用高温高压方法,在700~1 500 ℃、5.0 GPa条件下研究了VC陶瓷的微观结构演化、力学性能及抗氧化行为,结果表明:在700 ℃时,VC晶粒间存在大量微小孔隙,此时相对密度仅有91.5%(图8(a))。随着温度升高至1 000 ℃,晶粒尺寸缓慢增大,孔隙率略有下降,但仍未达到最致密的状态。温度达到1 100 ℃时,微观结构发生显著变化,晶界变得模糊,晶粒间界面结合紧密;SEM图像(图8(c))显示,孔隙几乎完全消失,相对密度急剧上升至99.8%(图8(a)),接近其理论密度(5.77 g/cm3);断裂模式由沿晶断裂转变为高密度穿晶断裂(图8(c)),表明晶间结合强度显著增强,裂纹扩展路径受阻;维氏硬度与断裂韧性分别达到30.4 GPa(29.4 N载荷下)和5.4 MPa·m1/2(图8(b));杨氏模量增大至545 GPa,接近其理论值(546 GPa),远高于传统烧结VC的杨氏模量(436 GPa[60]),其中杨氏模量由尼尔森模型(式(1))得到[45]。此优异力学性能源于高温高压烧结过程阻碍了原子的长程扩散并诱导了晶粒的塑性变形,使得样品内形成了高密度位错。图8(c)中的插图为典型维氏压痕的SEM图像。当温度超过1 100 ℃时,再结晶主导微观结构演化,异常晶粒生长导致晶粒尺寸骤增,并且由于晶界的过快迁移阻碍了孔隙排出,部分孔隙被包裹于晶内,阻碍了致密化,相对密度下降至约95%~97%,同时维氏硬度降至25.3 GPa,杨氏模量同步降低至509 GPa。另外,热重分析显示,力学性能最优的1 100 ℃、5.0 GPa烧结样品因高密度和低孔隙率,氧化起始温度约为655 ℃,抗氧化性显著强于900 ℃、5.0 GPa和1 300 ℃、5.0 GPa条件下的烧结样品。利用高温高压烧结,在VC中通过诱导晶粒塑性变形,达到高效致密化和微观结构调控,同时强化了其力学性能和抗氧化性。
-
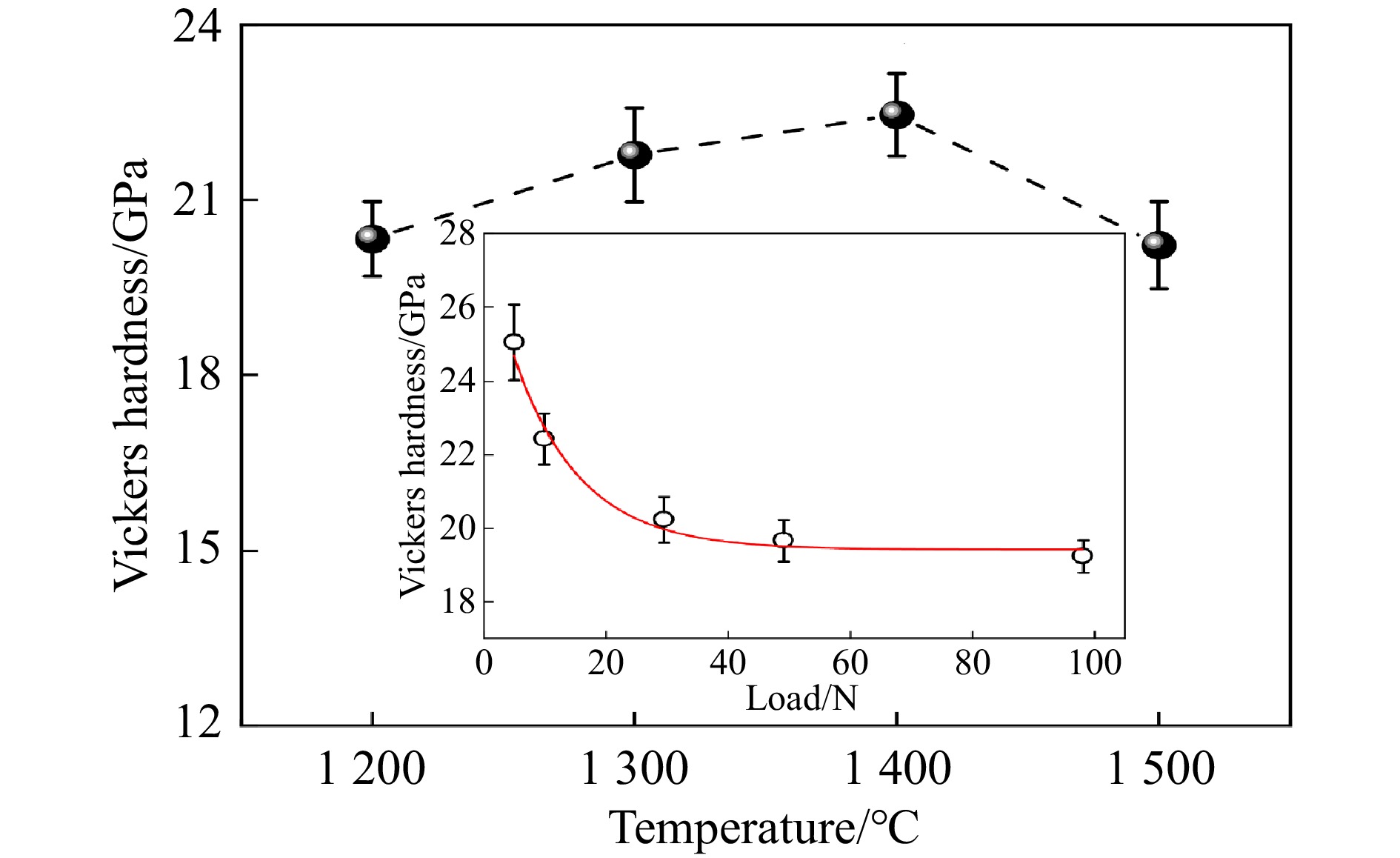
此前,碳化铌(niobium carbide, NbC)也通过放电等离子烧结[61]、热压烧结[11]、磁控溅射[35, 62]获得,但很少有针对纯NbC陶瓷烧结的研究。Liu等[63]在1 200~1 500 ℃、5.0 GPa条件下获得了纯NbC陶瓷,并且在1 400 ℃、5.0 GPa下得到相对密度高达99.4 %的NbC烧结体。高温高压处理后的微观结构显示,NbC烧结体颗粒间结合紧密,表明材料具有高强度。如图9所示,其收敛的维氏硬度高达19.2 GPa。热重分析显示,NbC的氧化起始温度为760 ℃,说明致密化有效抑制了氧气沿晶界和孔隙的渗透,延缓了氧化反应,表明其在高温环境下具有良好的抗氧化性能。高温高压为制备高性能无黏结剂NbC硬质陶瓷提供了有效途径,相较于传统工艺,其在硬度、韧性和密度方面的改进使其成为一种具有广阔应用前景的硬质材料。
-
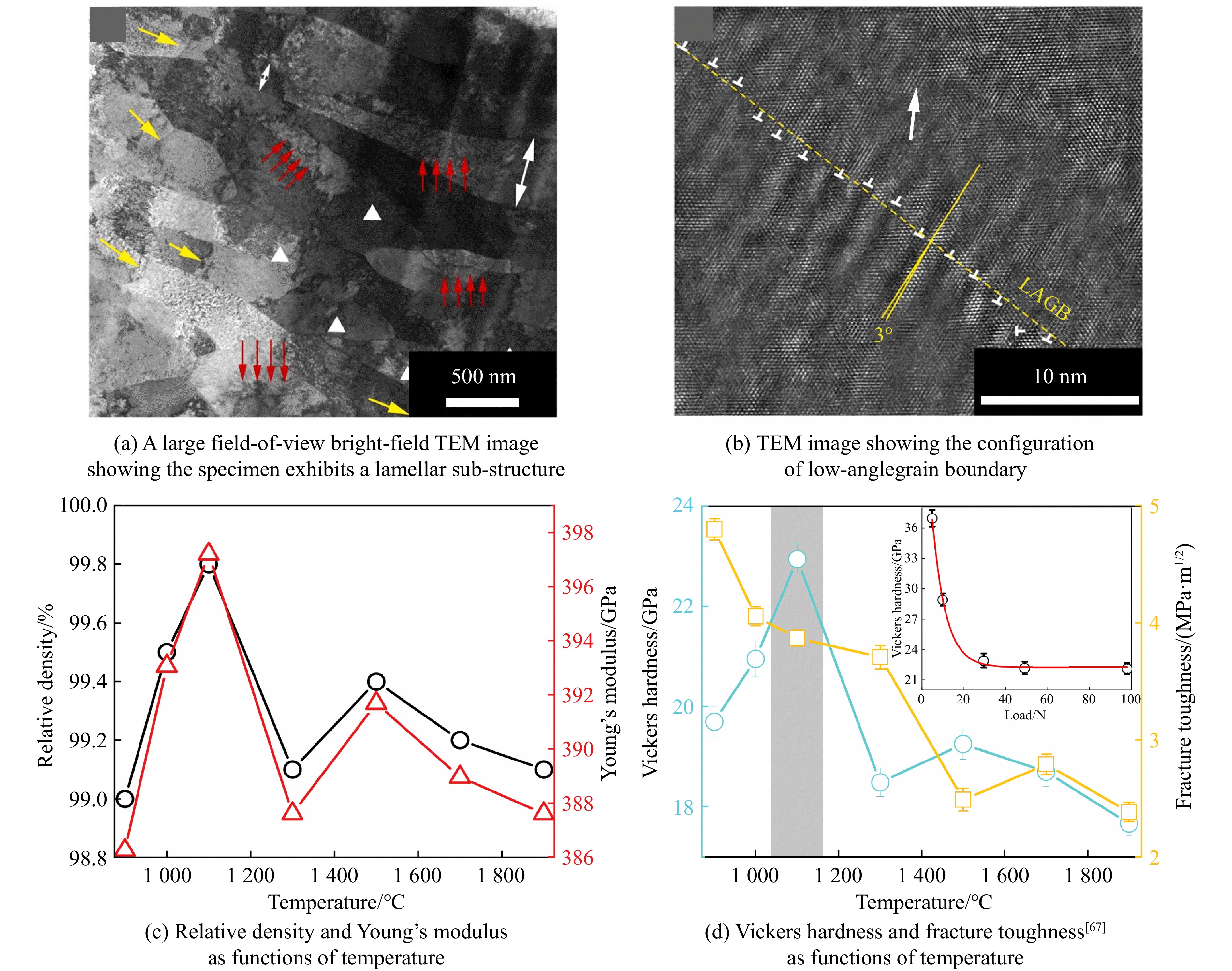
Wang等[64]通过快速加热固结得到了层状超导碳化物碳化钼(molybdenum hemicarbide, Mo2C),Díaz等[65]通过机械合金法制备了Mo2C粉体。关于Mo2C陶瓷制备的研究报道较少,Nino等[66]通过热压烧结,在1 550 ℃、50 MPa下合成了几乎完全致密的纯相多晶Mo2C陶瓷,其杨氏模量为398 GPa,维氏硬度为16 GPa。在超高压条件下,Liang等[67]利用高温高压方法,在900~1 700 ℃、15 GPa下制备了高性能Mo2C陶瓷,通过高温高压带来的高应变,产生了含有高位错密度的层状亚晶粒结构,并且在晶界处的位错最终演化为低角度晶界。从图10(a)可以看出,Mo2C微观结构以层状结构为主,还有独立的位错(黄色箭头)、位错阵列(白色三角形)和位于边界区域和晶粒内部的高密度位错(红色箭头)。大量高密度位错分布在片层边界处,可以阻止晶格位错的运动,位错几乎不沿晶界运动,使Mo2C表现出较高的强度,但同时削弱了其塑性[68];而晶内位错密度可以提高多晶材料的硬度和断裂韧性。另外,同一片层内的厚度分布不均(如白色双箭头所示),如同在金属冷轧过程中发现的现象[69],是退火过程中的动态边界迁移所致。图10(b)显示了采用透射电子显微镜(transmission electron microscope, TEM)得到的2个层状结构边界处(黄色虚线标记)的原子晶格。低角度晶界(low-angle grain boundaries, LAGB)如白色虚线所示,在<101>方向,上层的<102>晶面相对于下层偏转了约3°,“⊥”表示沿片层边界的高密度位错。这表明,在高压下,高应变引起的晶粒塑性变形产生了高密度位错的层状结构,而位错在层状边界处最终演化为低角度晶界。这些缺陷结构(位错和低角度晶界)导致了Mo2C的结构强化[67]。图10(c)展示了相对密度和杨氏模量与烧结温度的关系,其中杨氏模量由尼尔森模型(式(1))得到[45]。在1 100 ℃、15 GPa下得到的样品的相对密度高达99.8%,对应的杨氏模量为397 GPa。样品的相对密度在1 300 ℃时急剧下降到99.1%,这归因于塑性变形和再结晶,其中再结晶对相对密度的影响占主导地位。以上结果说明,高温高压方法诱导产生了塑性变形、高密度位错和低角度晶界,这种独特的微观结构阻止了层间滑移,对提高层状Mo2C的力学性能起着重要作用,使得在1 100 ℃、15 GPa条件下制备的样品具有较高的收敛硬度(23 GPa)和中等的断裂韧性(3.9 MPa·m1/2)。
-
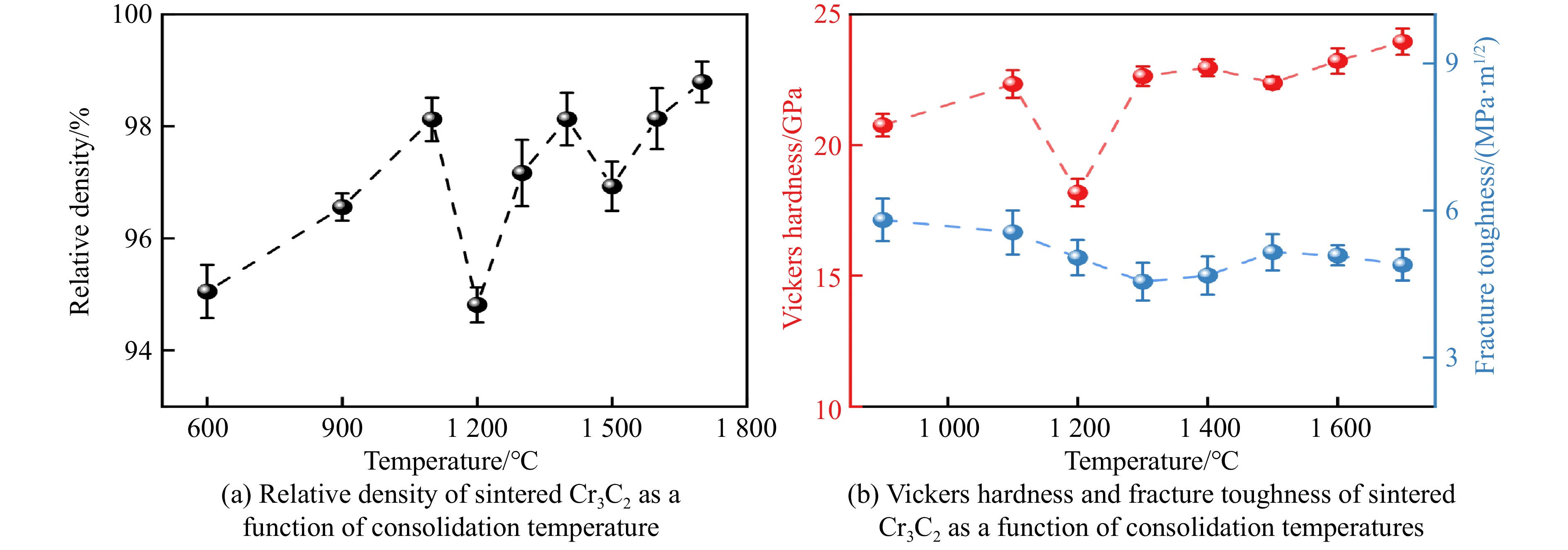
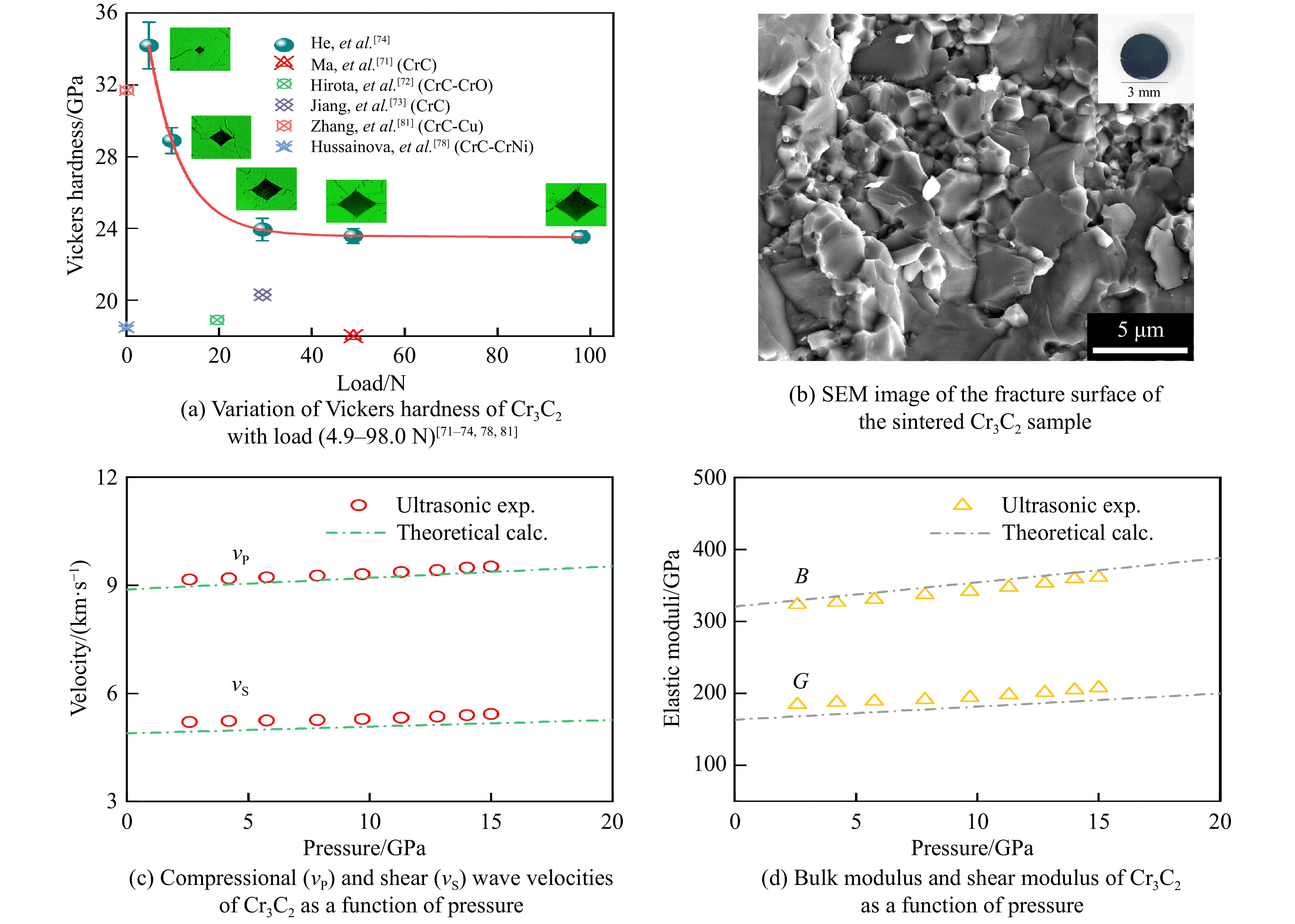
关于碳化铬(chromium carbide, Cr3C2)陶瓷的合成,Furukawa等[70]利用热压烧结和热等静压(hot-isostatically press, HIP)烧结方法,分别在1 300 ℃、40 MPa和1 330 ℃、150 MPa条件下获取了Cr3C2陶瓷,其维氏硬度和断裂韧性分别为18.5 GPa、5.6 MPa·m1/2和17.0 GPa、5.0 MPa·m1/2。Ma等[71]通过脉冲电流加压烧结(pulsed electric-current pressure sintering, PECPS),在727 ℃、100 MPa条件下烧结得到了硬度为18.0 GPa、断裂韧性为5.6 MPa·m1/2的致密陶瓷。Hirota等[72]和Jiang等[73]分别通过脉冲电流加压烧结,在1 300 ℃、30 MPa温压条件下获取了具有优质性能的Cr3C2陶瓷,其维氏硬度和断裂韧性分别为18.9 GPa和7.1 MPa·m1/2。在超高压力烧结中,He等[74]在15 GPa、25~1 700 ℃范围内烧结了Cr3C2陶瓷,当烧结温度达到1 200和1 500 ℃时,Cr3C2陶瓷的相对密度发生突降,与之对应的力学性能也同样呈现异常,如图11所示。这一异常源于晶粒细化与生长的竞争行为[74]:当温度较低时,晶粒在高压下受挤压破碎,形成更细的晶粒,细晶粒填充孔隙,使得材料致密度提高;随着温度升高,晶界扩散加速,晶粒快速生长,导致晶界处形成孔隙,材料致密度降低,同时相应的力学性能也降低。此外,He等[74]在1 700 ℃、15 GPa条件下烧结得到了相对密度为99.0%的致密陶瓷,如图12(a)所示,其维氏硬度在4.9 N加载时约为34.2 GPa,收敛硬度为24.0 GPa,断裂韧性为4.9 MPa·m1/2,经超高压合成的Cr3C2陶瓷的硬度比以往的报道高出17%~32%。烧结的Cr3C2陶瓷的断裂面形貌如图12(b)所示,可以看出,晶粒之间键合紧密,表现出大量穿晶断裂的断裂行为。
He等[74–75]通过高压超声测量装置研究了Cr3C2陶瓷在高压下的本征声速和弹性。图12(c)和图12(d)展示了其在高压下的声速、体积模量和剪切模量。Cr3C2在零压下的体积模量、剪切模量以及体积模量和剪切模量关于压力的导数分别为B0=319 GPa,G0=182 GPa,
$ {\partial B/\partial}p = 3.16 $ 和$ {\partial G/\partial}p= 1.82 $ ;杨氏模量E0 = 459 GPa,高于其他形式Cr3C2的杨氏模量,如薄膜、复合材料(300~390 GPa)[76–80],说明高温高压烧结合成的高致密的纯Cr3C2陶瓷展现出了材料的本征性质。 -
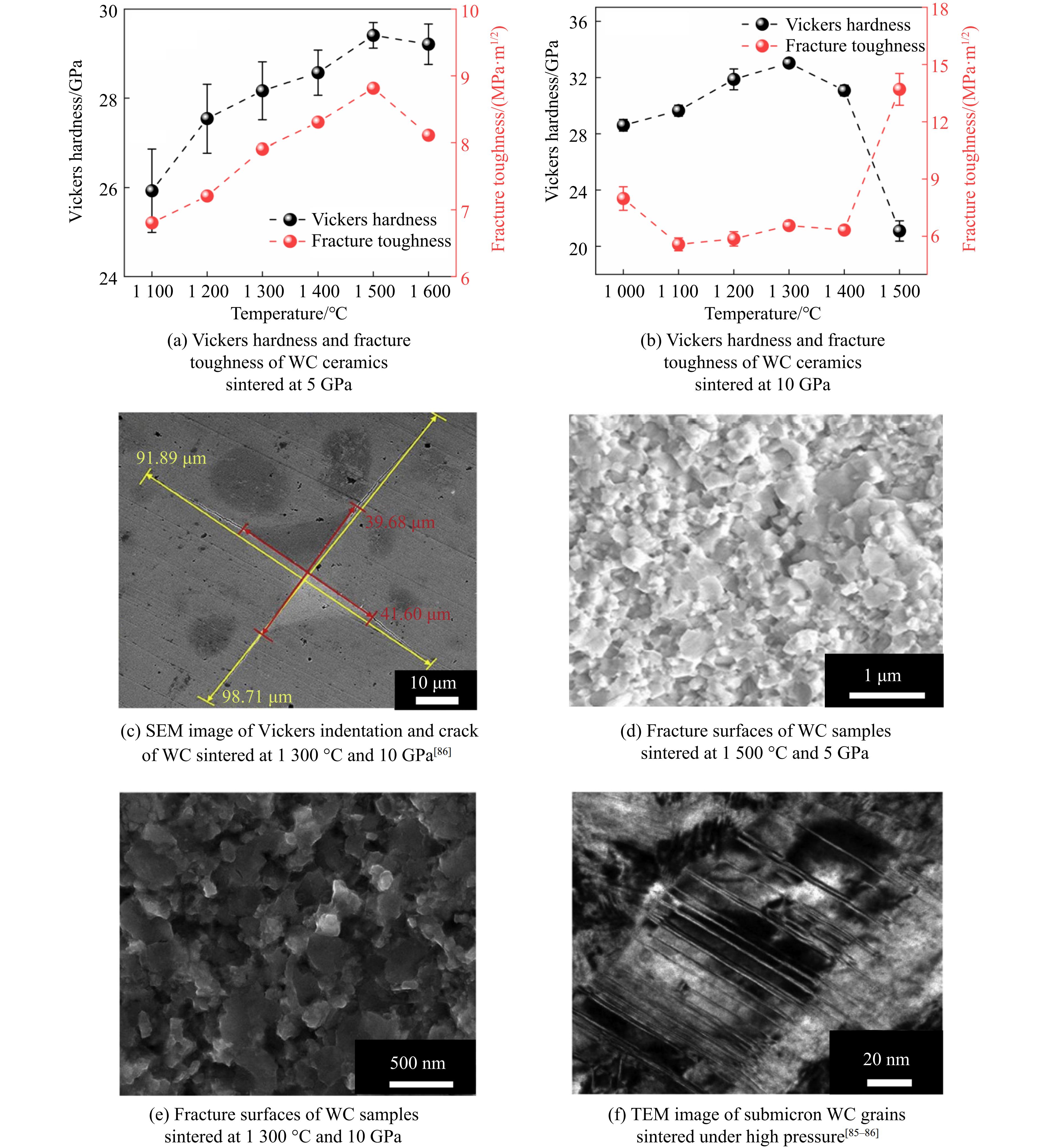
碳化钨(tungsten carbide, WC)由于其高硬度、高耐热性、优良的导电性、高熔化温度和高化学稳定性,被广泛用作工业加工领域中耐磨部件、切削工具和钻孔工具的常用材料。碳化钨常加入黏合剂金属钴(Co)烧结成WC-Co硬质合金。在实际应用中,WC-Co硬质合金的断裂韧性会随Co含量的增加而显著增大,而硬度则大幅降低[82]。此外,WC在黏结相中的溶解度很高,烧结温度越高,保温时间越长,WC的晶粒尺寸越大。由于晶粒尺寸与强度之间的霍尔-佩奇效应,WC的晶粒生长降低了烧结块状样品的硬度和断裂韧性[83–84]。对于纯相WC,利用高温高压方法可以达到同时提高硬度和断裂韧性的目的。如Ma等[85]采用高温高压方法,在5 GPa压力下研究了亚微米(约200 nm)晶粒尺寸的纯WC的烧结和力学行为,其高压下烧结的样品的力学性能与温度的关系如图13(a)所示。在5 GPa压力下,烧结温度为1 500 ℃时获得了相对密度较高(99.2%)且性能优异的WC陶瓷,具有较高的维氏硬度(29.3 GPa)和高断裂韧性(8.9 MPa·m1/2)。
Zhang等[86]对亚微米(约200 nm)晶粒尺寸的纯WC在10 GPa高压下进行了高温高压烧结,在1 300 ℃、10 GPa条件下得到了维氏硬度高达33 GPa、断裂韧性为6.6 MPa·m1/2的WC陶瓷,如图13(b)和图13(c)所示。从以上的烧结条件看来,更高的压力确实有助于降低烧结温度且提升性能。事实上,Chuvilʼdeev等[87]和Grasso等[88]也利用纳米级粉体,通过放电等离子烧结技术制备了纯相WC陶瓷,硬度分别为34和27 GPa。该结果与采用亚微米粉体通过高温高压烧结制备的WC陶瓷的硬度相当。这说明,除了细晶强化效应外,高温高压烧结还具有另一种强化机制,正如Ma等[85]和Zhang等[86]指出的,通过扫描电镜和透射电镜观察,发现烧结样品中引入了大量微缺陷(堆叠层错和孪晶),且烧结样品的晶粒尺寸几乎与初始粉体一致,如图13(d)和图13(f)所示。高压强化机制是高压使晶粒发生体积屈服,产生严重的塑性变形,并引入许多微观缺陷,缺陷可以阻碍位错滑移,从而提高其机械性能。
-
过渡金属碳化物的力学性能高度依赖于其微观结构特征(如晶粒尺寸、缺陷等)及致密化程度,而烧结工艺是影响这些特征的关键因素。目前,放电等离子烧结、高频感应加热烧结及高温高压烧结等先进技术被广泛应用于过渡金属碳化物的制备,通过调控热力学路径实现性能优化,如表1所示。其中:GPCS为气压燃烧烧结(gas-pressure combustion sintering),KIC为断裂韧性。可以看出,针对ⅣB~ⅥB族典型碳化物(如TiC、ZrC、NbC、VC等),采用不同烧结工艺得到的产物在弹性模量、维氏硬度、断裂韧性、相对密度及热稳定性等性能指标上呈现显著差异。特别值得注意的是,高温高压方法通过高压与高温的协同作用,能够有效抑制晶粒粗化并促进致密化,从而在多材料体系中实现性能突破。例如,采用高温高压方法在1 500 ℃、14 GPa条件下制备的TiC陶瓷的维氏硬度高达31.2 GPa,相对密度达到99.7%,显著高于无压烧结的20.3 GPa和95.7%。在1 300 ℃、5 GPa条件下制备的ZrC陶瓷不仅机械性能得到显著提升,热稳定温度也高达713 ℃,为极端环境应用提供了关键支撑。相较于其他制备工艺,高温高压方法的高压特性不仅提升了材料的力学性能,还通过位错强化机制增强了高温稳定性。因此,高温高压烧结技术在制备高性能过渡金属碳化物方面展现出显著优势,为其在极端环境下的应用开辟了新的可能性。
-
高温高压烧结为过渡金属碳化物陶瓷的合成提供了良好途径,从烧结温度和烧结时长来说大大降低了烧结难度,并且在压力的作用下可以抑制晶粒长大,提高致密化程度。高温高压烧结的强化机理之一是细晶强化,此外,过渡金属碳化物陶瓷在高压下发生严重的塑性变形,高应变引入大量微观缺陷,如高密度位错、孪晶、低角度晶界,这些缺陷可以阻碍位错的滑移,从而提高其机械性能。
目前,通过高温高压合成,过渡金属碳化物陶瓷的力学性能已经有所突破,结合其本身熔点高、机械强度高、化学稳定性好等优点,有望应用于机械加工、航空航天、聚变堆等领域。今后的研究将主要集中在以下3个方面。
(1) 探索烧结技术和烧结工艺。根据高温高压烧结机理,更高的压力和特别设置的烧结工艺有助于产生更多的缺陷,有望进一步提高陶瓷的力学性能。
(2) 提高抗氧化性。在实际应用中,过渡金属碳化物陶瓷将面临高温环境,在利用高温高压烧结保证其优异力学性能的前提下,能否进一步提升其抗氧化性,是关乎其能否产业化应用和能否拓展应用范围的前提。
(3) 开发高强韧的过渡金属碳化物陶瓷。通过对陶瓷进行成分设计和结构调控,开发高强韧的陶瓷,以适用于更多的应用环境。
典型过渡金属碳化物(ⅣB~ⅥB族)的超高压制备研究进展
Research Progress on the Ultra-High Pressure Preparation of Typical Transition Metal Carbides (Group ⅣB −ⅥB)
-
摘要: 过渡金属碳化物具有高硬度、高熔点、高电导率、耐腐蚀等优异的综合性能,在航空航天、切削加工等极端环境领域具有广阔的应用前景。由于过渡金属碳化物具有强共价键和低扩散系数,其烧结制备所需的温度极高,制备高致密度且性能优异的块体陶瓷具有挑战性。高温高压烧结方法具有可有效降低烧结温度、缩短烧结时间、抑制晶粒生长、提高致密化程度并保持物相纯净等优点。本文从高温高压合成角度,综述了数种典型过渡金属碳化物(ⅣB~ⅥB族)的制备、力学性能、微观机制的研究进展,总结并展望了过渡金属碳化物陶瓷的应用前景和未来发展方向。Abstract: Transition metal carbides (TMCs) exhibit exceptional properties, including high hardness, high melting point, excellent electrical conductivity, and corrosion resistance, making them promising candidates for extreme environments such as aerospace and cutting tools. However, the strong covalent bonding and low diffusion coefficients inherent to TMCs necessitate extremely high sintering temperatures, posing significant challenges for fabricating dense bulk ceramics with superior properties. The high pressure and high temperature (HPHT) sintering technique offers distinct advantages, effectively lowering sintering temperatures, reducing processing times, suppressing grain growth, enhancing densification, and preserving phase purity. This review summarizes recent advances in the HPHT synthesis, mechanical properties, and underlying mechanisms of several typical TMCs (Groups ⅣB to ⅥB). The application prospects and future research directions for TMC ceramics are also discussed and outlined.
-

-
表 1 典型过渡金属碳化物的烧结工艺参数、合成条件与性能的对比
Table 1. Comparison of sintering parameters, synthesis conditions, and performances of typical transition metal carbides
TMCs Synthetic method Synthesis condition Vickers
hardness/GPaE/GPa KIC/
(MPa·m1/2)Relative
density/%Thermal
stability/℃TiC SPS 1 650 ℃, 100 MPa[38–39] 25.7/27 97.9/95.1 HFIHS 80 MPa[16] 25.7 99 PS 1 700 ℃[40] 20.3 95.7 HPHT 1 500 ℃, 14 GPa[41] 31.2 4.2 99.7 ZrC SPS 1 850 ℃, 100 MPa[42] 20.4 376 1.8 98 1 800 ℃, 40 MPa[43] 17.6 3.3 95.5 PS 2 100 ℃[89] 8.9 94.4 HPHT 1 300 ℃, 5 GPa[44] 27.4 412 4.3 98.2 713 HfC SPS 2 200 ℃, 65 MPa[31] 19 500 98 2 300 ℃, 38 MPa[13] 10.2 283 2.9 85 HPS 1 900 ℃, 30 MPa[10] 5.79 1.88 89 HPHT 1 700 ℃, 15 GPa[32] 25.8 455 5.5 99.5 860 VC HPHT 1 100 ℃, 5 GPa[59] 30.4 544 5.4 99.8 655 NbC HPHT 1400 ℃, 5 GPa[63]19.2 7.7 99.4 TaC SPS 2 350 ℃, 38 MPa[13] 13.9 458 2.7 98 HPS 2 300 ℃, 30 MPa[52] 14 3 94 2 000 ℃, 40 MPa[54] 15.7 4.1 97 HFIHS 80 MPa[53] 22 5.1 96 HPHT 1 300 ℃, 5.5 GPa[56] 21 457 97.7 Cr3C2 HPS 1 300 ℃, 40 MPa[70] 18.5 5.6 HIP 1 330 ℃, 150 MPa[70] 17 5 GPCS 727 ℃, 100 MPa[71] 18 5.6 PECPS 1 300 ℃, 30 MPa[72] 18.9 7.1 98.9 HPHT 1 700 ℃, 15 GPa[74] 24 459 4.9 99 Mo2C HPS 1 550 ℃, 50 MPa[66] 16 400 100 HPHT 1 100 ℃, 15 GPa[67] 23 397 3.9 99.8 655 WC SPS 1 400 ℃, 80 MPa[88] 27 7.2 99.3 HPHT 1 500 ℃, 5 GPa[85] 29.3 8.9 99.2 1 300 ℃, 10 GPa[86] 33 6.6 -
[1] FAHRENHOLTZ W G, HILMAS G E. Ultra-high temperature ceramics: materials for extreme environments [J]. Scripta Materialia, 2017, 129: 94–99. doi: 10.1016/j.scriptamat.2016.10.018 [2] SAVINO R, DE STEFANO FUMO M, PATERNA D, et al. Aerothermodynamic study of UHTC-based thermal protection systems [J]. Aerospace Science and Technology, 2005, 9(2): 151–160. doi: 10.1016/j.ast.2004.12.003 [3] 董绍明, 王京阳, 倪德伟. 结构陶瓷——承载人类文明的基石 [J]. 无机材料学报, 2024, 39(6): 569–570. doi: 10.15541/jim20240000 DONG S M, WANG J Y, NI D W. Structural ceramics—the cornerstone of human civilization [J]. Journal of Inorganic Materials, 2024, 39(6): 569–570. doi: 10.15541/jim20240000 [4] 张幸红, 王义铭, 程源, 等. 超高温陶瓷复合材料研究进展 [J]. 无机材料学报, 2024, 39(6): 571–590. doi: 10.15541/jim20230609 ZHANG X H, WANG Y M, CHENG Y, et al. Research progress on ultra-high temperature ceramic composites [J]. Journal of Inorganic Materials, 2024, 39(6): 571–590. doi: 10.15541/jim20230609 [5] WOO Y C, KANG H J, KIM D J. Formation of TiC particle during carbothermal reduction of TiO2 [J]. Journal of the European Ceramic Society, 2007, 27(2/3): 719–722. doi: 10.1016/j.jeurceramsoc.2006.04.090 [6] UL-HAMID A. Microstructure, properties and applications of Zr-carbide, Zr-nitride and Zr-carbonitride coatings: a review [J]. Materials Advances, 2020, 1(5): 1012–1037. doi: 10.1039/D0MA00233J [7] LEVASHOV E A, MUKASYAN A S, ROGACHEV A S, et al. Self-propagating high-temperature synthesis of advanced materials and coatings [J]. International Materials Reviews, 2017, 62(4): 203–239. doi: 10.1080/09506608.2016.1243291 [8] BOLOKANG S, BANGANAYI C, PHASHA M. Effect of C and milling parameters on the synthesis of WC powders by mechanical alloying [J]. International Journal of Refractory Metals and Hard Materials, 2010, 28(2): 211–216. doi: 10.1016/j.ijrmhm.2009.09.006 [9] RAJKUMAR K, ARAVINDAN S. Microwave sintering of copper-graphite composites [J]. Journal of Materials Processing Technology, 2009, 209(15/16): 5601–5605. doi: 10.1016/j.jmatprotec.2009.05.017 [10] SILVESTRONI L, BELLOSI A, MELANDRI C, et al. Microstructure and properties of HfC and TaC-based ceramics obtained by ultrafine powder [J]. Journal of the European Ceramic Society, 2011, 31(4): 619–627. doi: 10.1016/j.jeurceramsoc.2010.10.036 [11] WOYDT M, MOHRBACHER H. The use of niobium carbide (NbC) as cutting tools and for wear resistant tribosystems [J]. International Journal of Refractory Metals and Hard Materials, 2015, 49: 212–218. doi: 10.1016/j.ijrmhm.2014.07.002 [12] KORKLAN N, HILMAS G E, FAHRENHOLTZ W G. Processing and room temperature mechanical properties of a zirconium carbide ceramic [J]. Journal of the American Ceramic Society, 2021, 104(1): 413–418. doi: 10.1111/jace.17442 [13] CEDILLOS-BARRAZA O, GRASSO S, NASIRI N A, et al. Sintering behaviour, solid solution formation and characterisation of TaC, HfC and TaC-HfC fabricated by spark plasma sintering [J]. Journal of the European Ceramic Society, 2016, 36(7): 1539–1548. doi: 10.1016/j.jeurceramsoc.2016.02.009 [14] BABAPOOR A, ASL M S, AHMADI Z, et al. Effects of spark plasma sintering temperature on densification, hardness and thermal conductivity of titanium carbide [J]. Ceramics International, 2018, 44(12): 14541–14546. doi: 10.1016/j.ceramint.2018.05.071 [15] KELLY J P, GRAEVE O A. Mechanisms of pore formation in high-temperature carbides: case study of TaC prepared by spark plasma sintering [J]. Acta Materialia, 2015, 84: 472–483. doi: 10.1016/j.actamat.2014.11.005 [16] SHON I J, KIM B R, DOH J M, et al. Consolidation of binderless nanostructured titanium carbide by high-frequency induction heated sintering [J]. Ceramics International, 2010, 36(6): 1797–1803. doi: 10.1016/j.ceramint.2010.03.007 [17] KIM H C, YOON J K, DOH J M, et al. Rapid sintering process and mechanical properties of binderless ultra fine tungsten carbide [J]. Materials Science and Engineering: A Structural Materials: Properties, Microstructure and Processing, 2006, 435/436: 717–724. doi: 10.1016/j.msea.2006.07.127 [18] KIM B R, WOO K D, YOON J K, et al. Mechanical properties and rapid consolidation of binderless niobium carbide [J]. Journal of Alloys and Compounds, 2009, 481(1/2): 573–576. doi: 10.1016/j.jallcom.2009.03.036 [19] TOTH L E. Transition metal carbides and nitrides [M]. New York: Elsevier, 2014. [20] YOUNG C, ZHANG C, LOGANATHAN A, et al. Densification and oxidation behavior of spark plasma sintered hafnium diboride-hafnium carbide composite [J]. Ceramics International, 2020, 46(10): 14625–14631. doi: 10.1016/j.ceramint.2020.02.263 [21] ZHANG J, MA S, ZHU J W, et al. Microstructure and compression strength of W/HfC composites synthesized by plasma activated sintering [J]. Metals and Materials International, 2019, 25(2): 416–424. doi: 10.1007/s12540-018-0190-8 [22] IRIFUNE T, KAWAKAMI K, ARIMOTO T, et al. Pressure-induced nano-crystallization of silicate garnets from glass [J]. Nature Communications, 2016, 7(1): 13753. doi: 10.1038/ncomms13753 [23] SOLOZHENKO V L, KURAKEVYCH O O, LE GODEC Y. Creation of nanostuctures by extreme conditions: high-pressure synthesis of ultrahard nanocrystalline cubic boron nitride [J]. Advanced Materials, 2012, 24(12): 1540–1544. doi: 10.1002/adma.201104361 [24] FENG L, LEE S H, WANG H L, et al. Synthesis and densification of nano-crystalline hafnium carbide powder [J]. Journal of the European Ceramic Society, 2015, 35(15): 4073–4081. doi: 10.1016/j.jeurceramsoc.2015.08.004 [25] LIANG H, FANG L M, GUAN S X, et al. Insights into the bond behavior and mechanical properties of hafnium carbide under high pressure and high temperature [J]. Inorganic Chemistry, 2021, 60(2): 515–524. doi: 10.1021/acs.inorgchem.0c02800 [26] HE R Q, FANG L M, HAN T X, et al. Elasticity, mechanical and thermal properties of polycrystalline hafnium carbide and tantalum carbide at high pressure [J]. Journal of the European Ceramic Society, 2022, 42(13): 5220–5228. doi: 10.1016/j.jeurceramsoc.2022.06.039 [27] KURBATKINA V V, PATSERA E I, LEVASHOV E A, et al. SHS processing and consolidation of Ta-Ti-C, Ta-Zr-C, and Ta-Hf-C carbides for ultra-high-temperatures application [J]. Advanced Engineering Materials, 2018, 20(8): 1701075. doi: 10.1002/adem.201701075 [28] GOLLA B R, MUKHOPADHYAY A, BASU B, et al. Review on ultra-high temperature boride ceramics [J]. Progress in Materials Science, 2020, 111: 100651. doi: 10.1016/j.pmatsci.2020.100651 [29] CEDILLOS-BARRAZA O, MANARA D, BOBORIDIS K, et al. Investigating the highest melting temperature materials: a laser melting study of the TaC-HfC system [J]. Scientific Reports, 2016, 6(1): 37962. doi: 10.1038/srep37962 [30] ZHONG Y, XIA X H, SHI F, et al. Transition metal carbides and nitrides in energy storage and conversion [J]. Advanced Science, 2016, 3(5): 1500286. doi: 10.1002/advs.201500286 [31] SCITI D, GUICCIARDI S, NYGREN M. Densification and mechanical behavior of HfC and HfB2 fabricated by spark plasma sintering [J]. Journal of the American Ceramic Society, 2008, 91(5): 1433–1440. doi: 10.1111/j.1551-2916.2007.02248.x [32] LIANG H, LIN W T, FANG L M, et al. Achieving dislocation strengthening in hafnium carbide through high pressure and high temperature [J]. The Journal of Physical Chemistry C, 2021, 125(43): 24254–24262. doi: 10.1021/acs.jpcc.1c08086 [33] LI B S, LIEBERMANN R C. Study of the Earth’s interior using measurements of sound velocities in minerals by ultrasonic interferometry [J]. Physics of the Earth and Planetary Interiors, 2014, 233: 135–153. doi: 10.1016/j.pepi.2014.05.006 [34] DURLU N. Titanium carbide based composites for high temperature applications [J]. Journal of the European Ceramic Society, 1999, 19(13/14): 2415–2419. doi: 10.1016/S0955-2219(99)00101-6 [35] NEDFORS N, TENGSTRAND O, LEWIN E, et al. Structural, mechanical and electrical-contact properties of nanocrystalline-NbC/amorphous-C coatings deposited by magnetron sputtering [J]. Surface and Coatings Technology, 2011, 206(2/3): 354–359. doi: 10.1016/j.surfcoat.2011.07.021 [36] OGUNTUYI S D, JOHNSON O T, SHONGWE M B. Spark plasma sintering of ceramic matrix composite of TiC: microstructure, densification, and mechanical properties: a review [J]. The International Journal of Advanced Manufacturing Technology, 2021, 116(1): 69–82. doi: 10.1007/s00170-021-07471-y [37] ONO T, ENDO H, UEKI M. Hot-pressing of TiC-graphite composite materials [J]. Journal of Materials Engineering and Performance, 1993, 2(5): 659–664. doi: 10.1007/BF02650054 [38] TEBER A, SCHOENSTEIN F, TÊTARD F, et al. Effect of SPS process sintering on the microstructure and mechanical properties of nanocrystalline TiC for tools application [J]. International Journal of Refractory Metals and Hard Materials, 2012, 30(1): 64–70. doi: 10.1016/j.ijrmhm.2011.06.013 [39] ABDERRAZAK H, SCHOENSTEIN F, ABDELLAOUI M, et al. Spark plasma sintering consolidation of nanostructured TiC prepared by mechanical alloying [J]. International Journal of Refractory Metals and Hard Materials, 2011, 29(2): 170–176. doi: 10.1016/j.ijrmhm.2010.10.003 [40] FU Z Z, KOC R. Pressureless sintering of submicron titanium carbide powders [J]. Ceramics International, 2017, 43(18): 17233–17237. doi: 10.1016/j.ceramint.2017.09.050 [41] WANG Z W, KOU Z L, ZHANG Y F, et al. Micrometer-sized titanium carbide with properties comparable to those of nanocrystalline counterparts [J]. Journal of Applied Physics, 2019, 125(16): 165901. doi: 10.1063/1.5087754 [42] XIANG M Y, XIE J J, JI W, et al. Low temperature consolidation for fine-grained zirconium carbide from nanoparticles with ZrH2 as sintering additive [J]. Journal of the European Ceramic Society, 2017, 37(8): 3003–3007. doi: 10.1016/j.jeurceramsoc.2017.03.002 [43] ACICBE R B, GOLLER G. Densification behavior and mechanical properties of spark plasma-sintered ZrC-TiC and ZrC-TiC-CNT composites [J]. Journal of Materials Science, 2013, 48(6): 2388–2393. doi: 10.1007/s10853-012-7024-8 [44] YANG P, PENG F, XIAO X, et al. Sintering pure polycrystalline zirconium carbide ceramics with enhanced mechanical properties under high-pressure and high-temperature [J]. Journal of the European Ceramic Society, 2025, 45(5): 117115. doi: 10.1016/J.JEURCERAMSOC.2024.117115 [45] NIELSEN L F. Elasticity and damping of porous materials and impregnated materials [J]. Journal of the American Ceramic Society, 1984, 67(2): 93–98. doi: 10.1111/j.1151-2916.1984.tb09622.x [46] SCITI D, GUICCIARDI S, NYGREN M. Spark plasma sintering and mechanical behaviour of ZrC-based composites [J]. Scripta Materialia, 2008, 59(6): 638–641. doi: 10.1016/j.scriptamat.2008.05.026 [47] KE B R, JI W, ZOU J, et al. Densification mechanism, microstructure and mechanical properties of ZrC ceramics prepared by high-pressure spark plasma sintering [J]. Journal of the European Ceramic Society, 2023, 43(8): 3053–3061. doi: 10.1016/j.jeurceramsoc.2023.02.038 [48] BALKO J, CSANÁDI T, SEDLÁK R, et al. Nanoindentation and tribology of VC, NbC and ZrC refractory carbides [J]. Journal of the European Ceramic Society, 2017, 37(14): 4371–4377. doi: 10.1016/j.jeurceramsoc.2017.04.064 [49] DOLLÉ M, GOSSET D, BOGICEVIC C, et al. Synthesis of nanosized zirconium carbide by a sol-gel route [J]. Journal of the European Ceramic Society, 2007, 27(4): 2061–2067. doi: 10.1016/j.jeurceramsoc.2006.06.005 [50] DA A Y, LONG F, WANG J L, et al. Preparation of nano-sized zirconium carbide powders through a novel active dilution self-propagating high temperature synthesis method [J]. Journal of Wuhan University of Technology Materials Science Edition, 2015, 30(4): 729–734. doi: 10.1007/s11595-015-1220-8 [51] ZHANG X H, HILMAS G E, FAHRENHOLTZ W G. Densification and mechanical properties of TaC-based ceramics [J]. Materials Science and Engineering: A Structural Materials: Properties, Microstructure and Processing, 2009, 501(1/2): 37–43. doi: 10.1016/j.msea.2008.09.024 [52] ZHANG X H, HILMAS G E, FAHRENHOLTZ W G, et al. Hot pressing of tantalum carbide with and without sintering additives [J]. Journal of the American Ceramic Society, 2007, 90(2): 393–401. doi: 10.1111/j.1551-2916.2006.01416.x [53] KIM B R, WOO K D, DOH J M, et al. Mechanical properties and rapid consolidation of binderless nanostructured tantalum carbide [J]. Ceramics International, 2009, 35(8): 3395–3400. doi: 10.1016/j.ceramint.2009.06.012 [54] REZAEI F, KAKROUDI M G, SHAHEDIFAR V, et al. Densification, microstructure and mechanical properties of hot pressed tantalum carbide [J]. Ceramics International, 2017, 43(4): 3489–3494. doi: 10.1016/j.ceramint.2016.10.067 [55] CHEN H H, LIANG H, LIU L X, et al. Hardness measurements for high-pressure prepared TaB and nano-TaC ceramics [J]. Results in Physics, 2017, 7: 3859–3862. doi: 10.1016/j.rinp.2017.10.006 [56] ZHANG Z G, LIANG H, CHEN H H, et al. Exploring physical properties of tantalum carbide at high pressure and temperature [J]. Inorganic Chemistry, 2020, 59(3): 1848–1852. doi: 10.1021/acs.inorgchem.9b03055 [57] SUN W G, KUANG X Y, LIANG H, et al. Mechanical properties of tantalum carbide from high-pressure/high-temperature synthesis and first-principles calculations [J]. Physical Chemistry Chemical Physics, 2020, 22(9): 5018–5023. doi: 10.1039/C9CP06819H [58] DODD S P, CANKURTRAN M, JAMES B. Ultrasonic determination of the elastic and nonlinear acoustic properties of transition-metal carbide ceramics: TiC and TaC [J]. Journal of Materials Science, 2003, 38(6): 1107–1115. doi: 10.1023/A:1022845109930 [59] CHEN J, PENG F, WANG Y P, et al. Mechanisms and mechanical properties of high-temperature high-pressure sintered vanadium carbide ceramics [J]. International Journal of Refractory Metals and Hard Materials, 2024, 118: 106483. doi: 10.1016/J.IJRMHM.2023.106483 [60] WU L L, YAO T K, WANG Y C, et al. Understanding the mechanical properties of vanadium carbides: nano-indentation measurement and first-principles calculations [J]. Journal of Alloys and Compounds, 2013, 548: 60–64. doi: 10.1016/j.jallcom.2012.09.014 [61] HUANG S G, VAN DER BIEST O, LI L, et al. Properties of NbC-Co cermets obtained by spark plasma sintering [J]. Materials Letters, 2007, 61(2): 574–577. doi: 10.1016/j.matlet.2006.05.011 [62] VOJVODIC A, HELLMAN A, RUBERTO C, et al. From electronic structure to catalytic activity: a single descriptor for adsorption and reactivity on transition-metal carbides [J]. Physical Review Letters, 2009, 103(14): 146103. doi: 10.1103/PhysRevLett.103.146103 [63] LIU F M, LIU P P, PENG F, et al. Hardness and compression behavior of niobium carbide [J]. High Pressure Research, 2017, 37(2): 244–255. doi: 10.1080/08957959.2017.1297810 [64] WANG X H, HAO H L, ZHANG M H, et al. Synthesis and characterization of molybdenum carbides using propane as carbon source [J]. Journal of Solid State Chemistry, 2006, 179(2): 538–543. doi: 10.1016/j.jssc.2005.11.009 [65] DÍAZ BARRIGA ARCEO L, OROZCO E, MENDOZA-LEÓN H, et al. Nanostructures obtained from a mechanically alloyed and heat treated molybdenum carbide [J]. Journal of Alloys and Compounds, 2007, 434/435: 799–802. doi: 10.1016/j.jallcom.2006.08.193 [66] NINO A, TANAKA A, SUGIYAMA S, et al. Indentation size effect for the hardness of refractory carbides [J]. Materials Transactions, 2010, 51(9): 1621–1626. doi: 10.2320/matertrans.M2010110 [67] LIANG H, HE R Q, LIN W T, et al. Strain-induced strengthening in superconducting β-Mo2C through high pressure and high temperature [J]. Journal of the European Ceramic Society, 2023, 43(1): 88–98. doi: 10.1016/j.jeurceramsoc.2022.09.031 [68] LU K, LU L, SURESH S. Strengthening materials by engineering coherent internal boundaries at the nanoscale [J]. Science, 2009, 324(5925): 349–352. doi: 10.1126/science.1159610 [69] DOHERTY R D, HUGHES D A, HUMPHREYS F J, et al. Current issues in recrystallization: a review [J]. Materials Science and Engineering: A Structural Materials: Properties, Microstructure and Processing, 1997, 238(2): 219–274. doi: 10.1016/S0921-5093(97)00424-3 [70] FURUKAWA M, SATO M, NAKANO O, et al. Hot isostatic pressing of chromium carbide [J]. Nippon Tungsten Review, 1989, 22: 73–82. [71] MA X F, TANIHATA K, MIYAMOTO Y. Gas-pressure combustion sintering and properties of Cr3C2 ceramic and its composite with TiC [J]. Journal of the Ceramic Society of Japan, 1992, 100(1160): 605–607. doi: 10.2109/jcersj.100.605 [72] HIROTA K, MITANI K, YOSHINAKA M, et al. Simultaneous synthesis and consolidation of chromium carbides (Cr3C2, Cr7C3 and Cr23C6) by pulsed electric-current pressure sintering [J]. Materials Science and Engineering: A Structural Materials: Properties, Microstructure and Processing, 2005, 399(1/2): 154–160. doi: 10.1016/j.msea.2005.02.062 [73] JIANG B L, KOU Z L, MA D J, et al. Mechanical behavior of the Cr3C2 compound at high pressure and high temperature [J]. Advanced Materials Research, 2015, 1120/1121: 1187–1193. doi: 10.4028/www.scientific.net/AMR.1120-1121.1187 [74] HE R Q, FANG L M, SUN J C, et al. Abnormal sintering behaviors of chromium carbide under high pressure and high temperature [J]. Journal of the European Ceramic Society, 2025, 45(1): 116822. doi: 10.1016/J.JEURCERAMSOC.2024.116822 [75] HE R Q, FANG L M, CHEN X P, et al. Experimental study of covalent Cr3C2 with high ionicity: sound velocities, elasticity, and mechanical properties under high pressure [J]. Scripta Materialia, 2023, 224: 115146. doi: 10.1016/J.SCRIPTAMAT.2022.115146 [76] OYAMA S T. The chemistry of transition metal carbides and nitrides [M]. London: Springer Dordrecht, 1996. [77] ZHAI W Y, GAO Y M, SUN L, et al. High pressure in-situ synthesis and physical properties of Cr3C2-Ni cermets [J]. Ceramics International, 2017, 43(18): 17202–17205. doi: 10.1016/j.ceramint.2017.09.145 [78] HUSSAINOVA I, JASIUK I, SARDELA M, et al. Micromechanical properties and erosive wear performance of chromium carbide based cermets [J]. Wear, 2009, 267(1): 152–159. doi: 10.1016/j.wear.2008.12.104 [79] JELLAD A, LABDI S, BENAMEUR T. On the hardness and the inherent ductility of chromium carbide nanostructured coatings prepared by RF sputtering [J]. Journal of Alloys and Compounds, 2009, 483(1/2): 464–467. doi: 10.1016/j.jallcom.2008.07.220 [80] SINGH V, DIAZ R, BALANI K, et al. Chromium carbide-CNT nanocomposites with enhanced mechanical properties [J]. Acta Materialia, 2009, 57(2): 335–344. doi: 10.1016/j.actamat.2008.09.023 [81] ZHANG L, PANG X L, GAO K W, et al. Mechanical properties of a bi-continuous Cu-Cr3C2 composite [J]. Materials Science and Engineering: A Structural Materials: Properties, Microstructure and Processing, 2015, 623: 4–9. doi: 10.1016/j.msea.2014.10.074 [82] KIM H C, OH D Y, SHON I J. Synthesis of WC and dense WC-xvol.%Co hard materials by high-frequency induction heated combustion method [J]. International Journal of Refractory Metals and Hard Materials, 2004, 22(1): 41–49. doi: 10.1016/j.ijrmhm.2003.12.002 [83] BAO R, YI J H. Densification and alloying of microwave sintering WC-8wt.%Co composites [J]. International Journal of Refractory Metals and Hard Materials, 2014, 43: 269–275. doi: 10.1016/j.ijrmhm.2013.12.010 [84] WANG X, FANG Z Z, SOHN H Y. Grain growth during the early stage of sintering of nanosized WC-Co powder [J]. International Journal of Refractory Metals and Hard Materials, 2008, 26(3): 232–241. doi: 10.1016/j.ijrmhm.2007.04.006 [85] MA D J, KOU Z L, LIU Y J, et al. Sub-micron binderless tungsten carbide sintering behavior under high pressure and high temperature [J]. International Journal of Refractory Metals and Hard Materials, 2016, 54: 427–432. doi: 10.1016/j.ijrmhm.2015.10.001 [86] ZHANG Y F, KOU Z L, WANG Z W, et al. Magic high-pressure strengthening in tungsten carbide system [J]. Ceramics International, 2019, 45(7): 8721–8726. doi: 10.1016/j.ceramint.2019.01.195 [87] CHUVIL'DEEV V N, BLAGOVESHCHENSKIY Y V, NOKHRIN A V, et al. Spark plasma sintering of tungsten carbide nanopowders obtained through DC arc plasma synthesis [J]. Journal of Alloys and Compounds, 2017, 708: 547–561. doi: 10.1016/j.jallcom.2017.03.035 [88] GRASSO S, POETSCHKE J, RICHTER V, et al. Low-temperature spark plasma sintering of pure nano WC powder [J]. Journal of the American Ceramic Society, 2013, 96(6): 1702–1705. doi: 10.1111/jace.12365 [89] ZHAO L Y, JIA D C, DUAN X M, et al. Pressureless sintering of ZrC-based ceramics by enhancing powder sinterability [J]. International Journal of Refractory Metals and Hard Materials, 2011, 29(4): 516–521. doi: 10.1016/j.ijrmhm.2011.03.001 -


 首页
首页 登录
登录 注册
注册



 下载:
下载: