HTML
-
Some materials which can absorb water or aqueous solution up to 20 g/g of its dry weight are considered as absorbents like paper, cotton and pulp. In the 1970's, at the department of agriculture of U.S. (Peoria, NRRL) a new material has been synthesized with sorption capacity of more than 100 times of its dry weight and called superabsorbent polymer (SAP) [1-3]. Superabsorbent polymers (SAPs) are hydrophilic in nature, loosely crosslinked that can absorb and retain water and aqueous solution [4-7]. Because of the extraordinary properties and environmental friendly nature of SAPs, they became the focus of interest of most of the researchers and were extensively used in the field of medical, hygiene and agriculture [8-10].
Gel strength, thermal stability and cost of production were still few major issues for these kinds of SAPs. Some researchers made important and reasonable attempts to modify their mechanical and thermal properties. Petroleum based superabsorbents polymers were expensive and responsible for serious environmental pollution. First SAP based on acrylic acid (AA) was prepared to reduce the cost of production and pollution problem, but the gel strength and thermal stability was still an issue [11]. Therefore clay based polymers have been paid much attention to improve thermal and mechanical properties [12-14]. Lin et al. made a wonderful attempt to synthesize the SAPs by graft-copolymerization between mica and montmorillonite with partially neutralized AA and achieved excellent absorbency and reasonable cost of production [15]. The reason of high attention towards polymer/clay superabsorbent polymer composites (SAPCs) was because of low production cost and high thermal stability [16]. The sorption capability and synthesis of poly(acrylic acid)/attapulgite superabsorbent composite were reported in various studies [17, 18] which clearly show the enhanced water absorbency of SAPCs in distilled water. It has also been clearly reported that introduction of clay to SAPs fairly improved its gel strength, thermal stability and water absorbency, but limited information was available about the effect of kind and type of clay on the properties of corresponding SAPCs. Different clays, such as montmorillonite [19], attapulgite [20], mica, sercite [21], and bentonite [22] were used for the preparation of SAPCs. In the above mentioned strategies and techniques, production cost, thermal stability, enhanced water absorbency or retention ability were still some major concerns needed to be addressed.
Here we report the development of SAPCs with the following positive features. Cost of production has been greatly reduced by saving energy and using more clay and less chemical contents in feeding ratios. Energy is saved by decreasing the polymerization time and by exploiting the high crosslinking ability of China clay and by use of initiator and catalyst. Polymerization time was decreased to 15 min while the reaction temperature was reduced to 50 ℃. High absorbency of water, aqueous, and salt solution, and enhanced water retention ability were obtained by optimizing the feeding ratios of all the reactants. High thermal stability was also achieved because of more stable composition of China clay and appropriate grafting ratio. Schematic illustration of SAPC particles and their fabrication is shown in Scheme 1. SAPCs were prepared by graft copolymerization [21]. Solution of
$N$ ,$N$ -methylenebisacrylamide (MBA) as crosslinker, aqueous solution of potassium persulfate (KPS) as initiator, and sodium sulfite solution was used as a catalyst. To know the effect of amount of China clay and amount of AA on water absorbency of SAPCs, different samples were made and investigated. The resulting SAPCs were extensively characterized with Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), X-ray diffraction (XRD), and thermogravimetric analysis (TGA). SEM confirmed the surface morphology while the grafting ratio was investigated by FTIR. Formation of single phase material and crystallinity of SAPC were confirmed by XRD, which indicated that all the peaks were properly indexed and there was no sign of any impurity or second phase in these composites. Thermal stability of the SAPC with different clay content was determined by TGA. The resulting polymer composites showed high retention capacity for water and salt solution, high water absorbency, mechanically and thermally stable, environmental friendly and nontoxic in nature. These aforementioned properties could make these SAPCs be used in baby diapers, sanitary towels, in packing materials and in the field of agriculture.
-
Fine powder of China clay was used after being passed through 100-mesh screen. Acrylic acid with 99% purity was obtained from Sigma-Aldrich Company. Ultra-pure grade of
$N$ ,$N$ -methylenebisacrylamide was purchased from Amresco and Applichem (Czech). Potassium persulfate, sodium sulfite and sodium hydroxide were purchased from Riedel-de-Haen (Czech). All chemicals were used as received. -
Graft copolymerization reaction was used to synthesize SAPCs [21-23]. Series of samples with different amount of clay and AA were prepared by using well equipped three-neck round bottomed flask. In a typical process, China clay (9 g) mixed with distilled water was charged into a flask heated in water bath under agitating. When the temperature reaches 50 ℃, acrylic acid (3 g) dissolved in distilled water (partially neutralized by NaOH to pH=6) along with the solution of
$N$ ,$N$ -methylenebisacrylamide (0.5 g) as crosslinker, followed by the addition of solution of potassium persulfate (0.25 g) as initiator and sodium sulfite (0.25 g) solution as a catalyst. In nitrogen atmosphere, solution was stirred continuously for 10-15 min till the polymerization completed. Reaction product (SAPCs) was filtered, washed and dried in the oven at 50 ℃ till the weight was constant. -
To know the effect of different parameters on water absorbency and retention capability, the optimization of reaction has been carried out. Optimum amount of China clay by weight percent (20%, 30%, 40%, 45%, 50%, and 60%), AA by weight percent (25%, 30%, 35%, 40%, 45%, and 50%), crosslinker by weight percent (0.1%, 0.2%, 0.3%, 0.4%, 0.5%, 0.6%, 0.7%, 0.9%, 1%) and initiator by weight percent (0.1%, 0.2%, 0.3%, 0.4%, 0.5%, 0.6%, 0.7%, 0.9%, 1%) were studied by varying the one parameter while keeping all others constant.
-
FTIR analysis was performed by using Thermo Nicolet Avatar 320 spectrophotometer (USA). X-ray diffraction (XRD) analysis was carried out with X-ray Diffractometer SIEMENS D5000 with Cu anode, scanning 2
$\theta$ from 10$^{\circ}$ to 70$^{\circ}$ at 0.05$^{\circ}$ /min, running at 40 kV and 30 mA. The surface morphology of the SAPCs was investigated on a FEI/EO QUANTA (Holland) SEM, performing at an accelerating voltage of 10 kV. Before the SEM examination the samples were coated with a thin layer of gold. TGA of samples were studied on a thermogravimetric analyser (METTLER TELEDO TGA/ASDA851e) at a scanning rate of 10 ℃/min with a temperature range of 30-850 ℃ using a dry nitrogen purge at a flow rate of 80 mL/min. -
Water and salt solution absorbency of SAPCs was determined by the following method: 1 g of different SAPC samples were immersed in beaker containing 500 mL of distilled water or 1% NaCl solution for 24 h. Afterward the swollen samples were filtered through 100 mesh screen to remove unabsorbed water and reweighed. The water absorbency (
$Q$ ) was calculated using the following equation:here
$W_2$ and$W_1$ are the weight of the swollen and dry sample respectively. -
The ability to retain water and salt solution even at high pressure or temperature was determined by centrifuging the swollen sample at 5000 r/min for 1 h and also by keeping the swollen sample in oven at 50, 60, and 100 ℃ for 12-48 h.
A. Materials
B. Synthesis of SAPCs
C. Optimization of reaction
D. Characterizations and analysis
E. Measurement of absorbency
F. Measurement of retention capacity
-
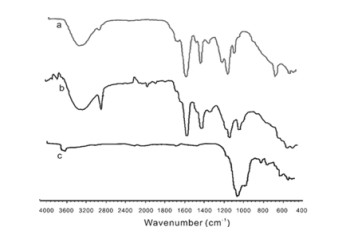
The FTIR spectra of poly acrylic acid, SAPC, and China clay are shown in FIG. 1. FIG. 1(a) shows the FTIR spectra of poly acrylic acid, which indicates the presence of absorption bands at 3403 cm
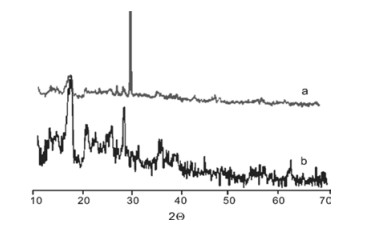
$^{-1}$ corresponding to stretching of OH group, 1565 cm$^{-1}$ is the asymmetric stretching of COO$^-$ group, 1400 and 1112 cm$^{-1}$ correspond to symmetric stretching of COO$^-$ and OH bands, respectively, which confirms the formation of poly acrylic acid [14]. FIG. 1(b) represents the FTIR spectra of SAPCs. The characteristic peak at 3670 cm$^{-1}$ corresponds to OH, peak at 3730 cm$^{-1}$ could arise from strong Al-O-H stretching, peak at 2925 cm$^{-1}$ is the CH stretch and 1120 cm$^{-1}$ is the -CO-O stretching of acrylate unit. The band at 1556 cm$^{-1}$ is from the COOH stretching, and 1400 cm$^{-1}$ is from symmetric stretching of COO$^-$ [22]. Peaks at 1040 and 517 cm$^{-1}$ are the Si-O stretching and O-Si-O stretching, respectively, arising from China clay as verified by FIG. 1(c), which confirmed China clay in SAPCs [23, 24]. The absorption bands in FIG. 1(c) at 1000 cm$^{-1}$ arise from Si-O-Si. The bands at 900 and 520 cm$^{-1}$ are the in-plane Si-O stretching and O-Si-O stretching, respectively [25] (FIG. 1(c)).FIG. 2 shows XRD patterns of China clay and SAPCs. FIG. 2 (a) shows that China clay demonstrated two characteristic peaks at 2
$\theta$ of 16.9$^{\circ}$ and 29.7$^{\circ}$ (correspondent to 5.2 and 3.0 Å in$d$ -spacing). The latter is correspondent to the interlayer distance. In SAPC, the interlayer refraction peak shifted to 2$\theta$ of 27.9$^{\circ}$ (or 3.2 Å in$d$ -spacing). These data indicate that polymerization of acrylic acid can increase the$d$ -space by entering into the silicate sheets. These results are coherent with Zhang's work which shows similar behaviour that monomers can enter into the layers of clay and expand the spaces during the formation of SAPC [23]. -
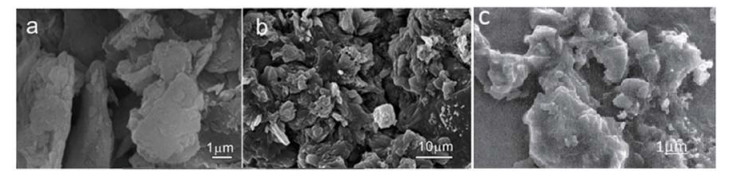
The morphologies of China clay, SAPC with 45% of clay contents and polyacrylic acid are shown in FIG. 3. FIG. 3(a) displays a smooth, tight and non-porous surface of clay and FIG. 3(c) also exhibits the non-porous surface with large particle size for polyacrylic acid with 0% of China clay. After intercalation of polyacrylic acid with 45% of China clay, the surface was modified to spongy and porous, as shown in FIG. 3(b), which facilitated the water permeation into the polymeric network. Moreover, the decrease in particle size in SAPC also provides more surface area and high sorption capacity and also small particles arrange themselves in such a way to create spaces among them which facilitate water absorption by capillary action method. Further decrease in particle size could decrease sorption capacity because the crosslinking density increases [21, 22].
-
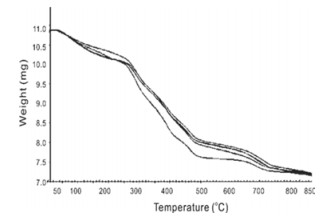
FIG. 4 shows the thermogravimetric analysis of the SAPCs with different clay contents. Three-stage thermal disintegration is shown in SAPCs. First weight loss is due to loss of moisture in the temperature range of 70-120 ℃. Second sharp weight loss takes place within the temperature range of 290-490 ℃ because of the thermal breakdown of amide groups of crosslinker on the network, while the third stage is indicated by the thermal disintegration of the backbone of polymer chains, which causes further gradual weight losses at 680 and 700 ℃ [24]. It has been clearly indicated that China clay micro-particles in composite network can tremendously boost the overall thermal stability of the system.
-
Swollen SAPC samples with water and 1% NaCl solution were placed in centrifuging machine at 5000 r/min for 1 h and showed an excellent water retention ability, and retained up to 98% of the distilled water and 95% of NaCl solution. Moreover, these swollen SAPC samples which were placed in oven at 60 ℃ and 100 ℃ for 12 h retained 85% and 60% of the distilled water and 90% and 72% of NaCl solution, respectively. Retention ability of SAPCs at 50 ℃ was remarkably high and calculated above 90% even for 48 h for both with distilled water and NaCl solution.
-
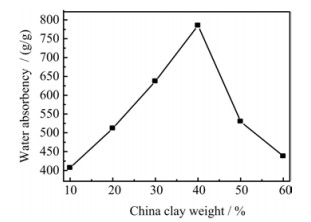
Optimum amount of China clay and acrylic acid for water absorbency was also determined. Effect of the amount of China clay on water absorbency of SAPC is shown in FIG. 5. Water absorbency increased from 10% to 40% of the clay contents and then decreased with further increase of clay. This result is because that clay particles fill the polymeric network and also cause more grafting with polymer structure to increase the density and complexity of the polymers. Therefore less spaces are available for water to permeate and hence water absorbency is decreased. These results show the similar behaviour to that reported by Li et al. [20] for attapulgite and Gao et al. [19] for montmorillonite.
-
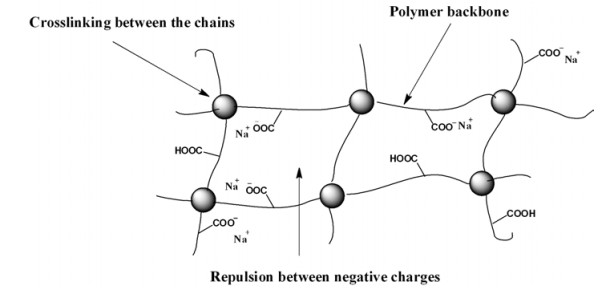
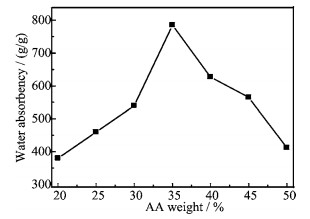
FIG. 6 shows the effect of percentage of acrylic acid on water absorbency where increase in absorbency has been shown in the range of acrylic acid percentage between 20% and 35%. These results are due to the presence of two hydrophilic groups (-COOH and -COONa groups) in the backbone of the polymers. When these groups interact in the suitable ratio, the repulsion among the ions decreases which fabricates the higher water absorbency [14].
A. FTIR Analysis
B. SEM analysis
C. TGA analysis
D. Water and salt solution retention test
E. Effect of China clay on water absorbency
F. Effect of acrylic acid on water absorbency
-
China clay/polyacrylic acid superabsorbent polymer composite was successfully synthesized, characterized and optimized. Novel SAPC has shown high absorbency of water (785 g/g) and 1% NaCl solution (102 g/g) and above 90% retention ability at 50 ℃. High thermal stability and gel strength have been achieved by using novel China clay as inorganic filler. Cost of production has been reduced by using less chemical contents and more China clay as an indigenous and economical resource and also by using the conditions where polymerization reaction takes place in less time and at relatively low temperature. Being non-toxic in nature, economical, high sorption capacity and high thermal stability make the SAPCs suitable to be used in baby diapers, as a desiccant, for adsorption of heavy metals and agricultural purposes.

 首页
首页 登录
登录 注册
注册





 DownLoad:
DownLoad: