-
Nowadays, commercial lithium-ion batteries (LIBs) are widely used for energy storage in our daily lives because of their high energy density and good cycling performance. However, safety and environmental issues, which arise from inflammable and toxic organic electrolytes, hinder their large-scale applications in some fields[1-4]. In contrast, aqueous sodium-ion batteries employ non-toxic aqueous electrolyte that is environmentally friendly and improves the safety of the batteries[5-8]. Besides, worldwide sodium reserves are much larger than lithium reserves, which could potentially reduce the cost of the sodium-ion batteries. Hence, high-performance aqueous sodium-ion batteries can be considered a promising alternative in some specific large-scale applications.
Unfortunately, the energy density of the aqueous sodium-ion batteries is lower than that of LIBs. Both the narrow operational potential window and the limited capacity of the electrodes restrict the energy density of the batteries. Broadening the potential window would lead to either side reactions involving the aqueous electrolyte or irreversible phase transition of the electrodes. Therefore, exploring high-capacity electrodes at this limited potential window would be a more efficient in enhancing energy density of the batteries. In the search for high-capacity Na-ion cathodes, many types of active materials have been developed, such as oxides, polyanions, and Prussian blue analogs[9-18]. Among them, Na0.44MnO2 has drawn researchers’ attention due to its large and stable S-shape and O-shape tunnels which facilitate Na ion insertion and extraction. The theoretical capacity of Na0.44MnO2 cathode is as high as 121 mAh/g. Besides, Mn-based oxides are low cost and environmentally benign materials[19-20]. Whitacre’s work firstly demonstrated that a Na0.44MnO2 cathode could offer a long cycle life with a cycling capacity of 30-40 mAh/g at lower rates (0.2 C and 1.2 C) when charged and discharged from between −0.1 V and 0.7 V. This capacity would decrease to less than 20 mAh/g if the cathodes were cycled at a high rate of 18 C[21]. Low electrical conductivity or low Na-ion diffusivity are considered as possible causes of poor cycling stability and rate capability. Increasing the interfacial area between the electrolyte and the electrode by decreasing the size or dimension of the Na0.44MnO2 cathode would be available to improve the conductivity and the Na-ion diffusivity.
In this work, Na0.44MnO2 with different particle sizes are prepared to investigate the size effect on its cathode performance. Among these Na0.44MnO2, the nanorod one is found improved performance. By using α-MnO2 nanoflakes as precursors, the Na0.44MnO2 nanorods were successfully prepared with a width of 200 nm. Such a size could enhance the electrical conductivity and the Na-ion diffusivity of the cathode. The as-prepared cathode shows a higher cycling capacity and lower polarization at a low rate of 1 C and a high rate of 5 C. The initial discharging capacity of Na0.44MnO2 nanorods cathode reaches 60 mAh/g at 1 C, with 55 mAh/g retained after 200 cycles. A high capacity of 47 mAh/g is delivered after 200 cycles at a high rate of 5 C. The high stability of thin Na0.44MnO2 nanorods is due to the stable morphology and crystal structure after long cycles.
-
Synthesis of Na0.44MnO2 nanorods. KMnO4 solution (0.01 mol/L) was heated at 100℃. Cetrimonium bromide (CTAB) solution (0.05 mol/L) was heated at 140℃. 100 mL of KMnO4 solution was injected into 100 mL of CTAB solution. The color of the obtained solution gradually turned from purple to brown. 500 mL of acetone was added to the solution and brown precipitates were obtained. The precipitates were washed with deionized water by centrifugation and dried at 80℃ for 24 h. The dried powder was added to 30 mL aqueous NaBH4 solution. The mixture was kept stirring at room temperature for 24 h. The obtained power was washed with deionized water by filtration and dried at 80℃ for 24 h. The dried powder was mixed and ground with NaHCO3 in a mass ratio of 2:1, and then heated in air at 500℃ for 5 h. After cooling down, the obtained powered was ground again and then heated at 900℃ in air for 12 h.
Synthesis of Na0.44MnO2 microrods. The synthesis procedure was the same as that for Na0.44MnO2 nanorods, except 0.1 mol/L KMnO4 solution was used, instead of 0.01 mol/L.
Synthesis of Na0.44MnO2 bulk. Mn2O3 and NaHCO3 were mixed and ground with 10% excess of Na in stoichiometry with the molar ratio of manganese atoms to sodium atoms as 1:0.484. The mixture was heated in air at 500℃ for 5 h. After cooling down, the obtained powered was ground again and then heated at 900℃ in air for 12 h.
Material Characterizations. X-ray diffraction (XRD) patterns were collected on a Panalytical XPert Pro X-ray diffractometer with Cu Kα radiation. Field emission scanning electron microscopy (FESEM) analysis was carried out on ZEISS Supra 55. High-resolution transmission electron microscopy (HRTEM) analysis was carried out using a JEOL 2100F at 200 kV. N2 (77 K) adsorption test was conducted on an ASAP Tristar Ⅱ 3020. Brunauer−Emmett−Teller (BET) was used for the surface area determination. Inductively coupled plasma optical emission spectrometer (ICP-OES) test was conducted on a PerkinElmer@Optima 8300.
Electrochemical Measurements. Three-electrode system was used for the evaluation of the Na0.44MnO2. The working electrode was prepared by mixing Na0.44MnO2, single-walled carbon nanotube (SWCNT), and polytetrafluoroethylene (PTFE) at 8:1:1, followed by pressing the mixture on a 4 cm2 Ni foam using a hydraulic press machine with a pressure of 10 MPa. The active mass loading was about 30 mg. A Pt foil was employed as a counter electrode and an Ag/AgCl (saturated NaCl) electrode was employed as a reference electrode. The electrolyte was 1 mol/L Na2SO4 in deionized water. Cyclic voltammetry (CV) measurement was conducted in a voltage range of −0.2 V to 0.8 V vs. Ag/AgCl at a scan rate of 0.1 mV/s. For galvanostatic charge-discharge (GCD) tests, the voltage window was controlled between −0.1 V and 0.7 V. 1 C corresponds to 121 mA/g in this work. Electrochemical impedance spectroscopy (EIS) was tested in the frequency range of 10 mHz to 100 kHz, and the perturbation amplitude is 5 mV. CV, GCD, and EIS were all measured on a Biological potentiostat electrochemical station.
-
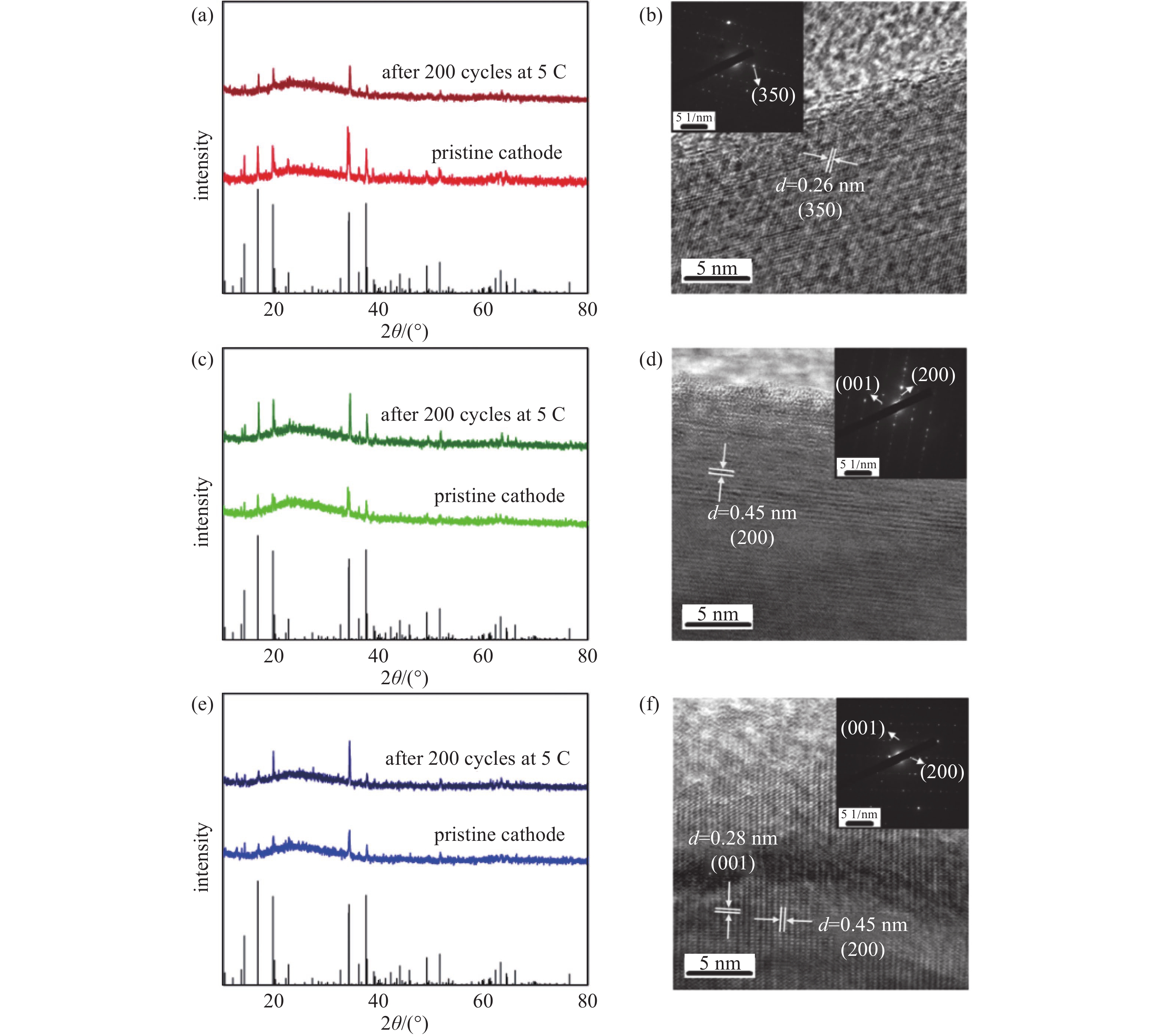
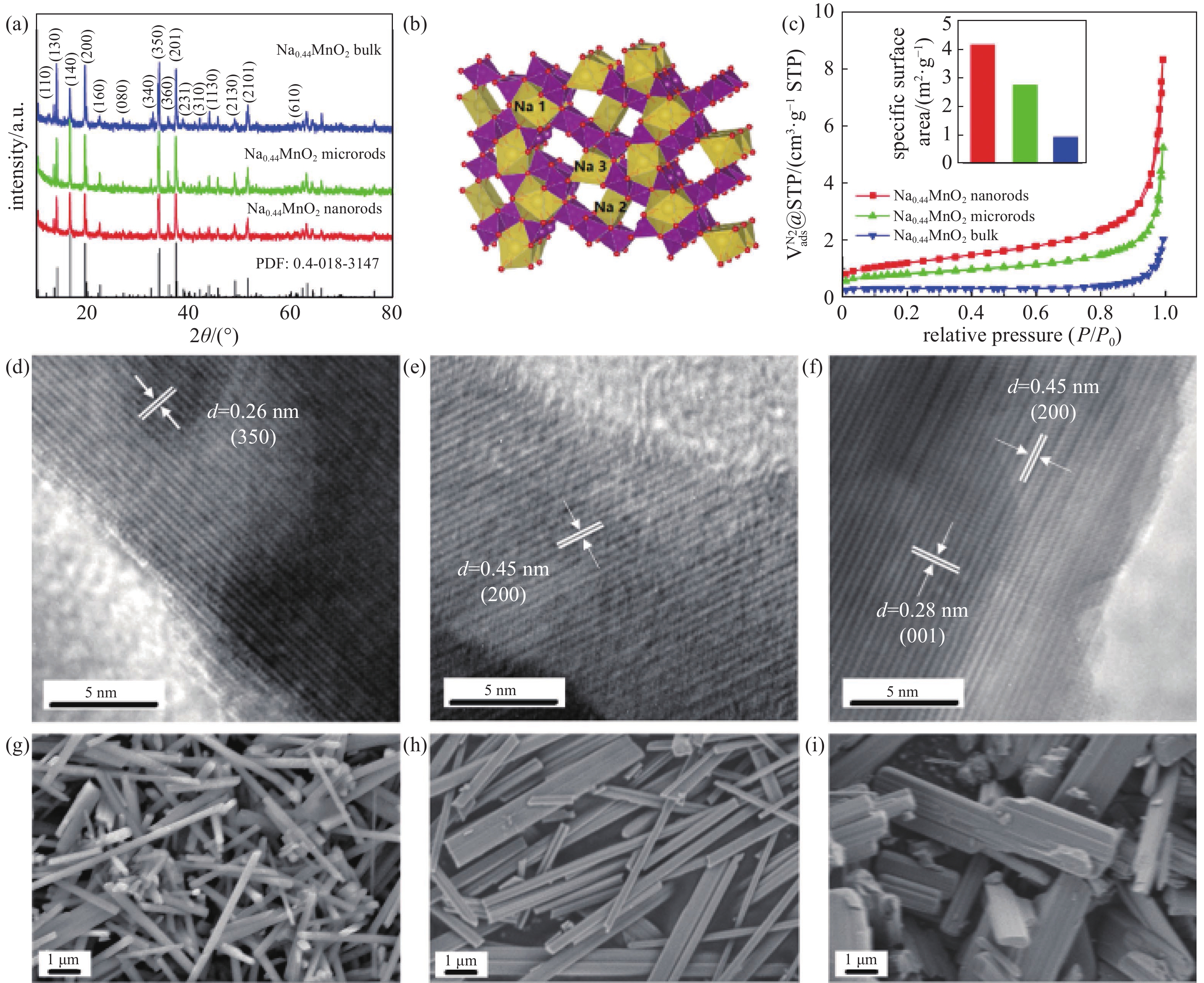
XRD was used to determine the structure of the prepared materials (Fig. 1(a)). All the diffraction peaks of the three materials can be assigned to the orthorhombic Na0.44MnO2 phase (Fig. 1(b)) [23]. The diffraction peaks are sharp and intense, which indicates the high crystallinity of the materials. HRTEM analysis shows interplanar spacing of 0.26 nm (Fig. 1(c)) and 0.45 nm (Fig. 1(d)) for Na0.44MnO2 nanorods and microrods, respectively. They correspond to (3 5 0) and (2 0 0) crystal planes of orthorhombic Na0.44MnO2, respectively. For Na0.44MnO2 bulk (Fig. 1(f)), interplanar spacings of 0.28 nm and 0.45 nm were determined, corresponding to (0 0 1), and (2 0 0) crystal planes, respectively. The ratios of Na/Mn in the three prepared materials were examined by ICP-OES (Table 1). They are all very close to 0.44. These demonstrate all the three prepared materials are Na0.44MnO2 with an orthorhombic structure.
The morphology and dimensions of the samples can be observed from SEM images displayed in Fig. 1(g), (h), and (i). Na0.44MnO2 bulk show varying dimensions with a wide size distribution. The bulk have lengths of 3−10 μm and widths of 0.5−1 μm. Na0.44MnO2 microrods have a smaller dimension than Na0.44MnO2 bulk, with a narrow size distribution and regular shapes. Its widths are smaller than 0.5 μm. A uniform rod shape can be observed in images of Na0.44MnO2 nanorods with widths of about 200 nm. The lengths of the nanorods range from 2 to 10 μm. The difference in morphology between the three samples may be caused by the using of CTAB as a surfactant. Na0.44MnO2 nanorods are prepared by using the largest CTAB/KMnO4 ratio, and its widths are the smallest. Na0.44MnO2 microrods are prepared by using the smaller CTAB/KMnO4 ratio, and its widths are larger than Na0.44MnO2 nanorods. CTAB is not needed when preparing Na0.44MnO2 bulk. The precursor of Na0.44MnO2 bulk, Mn2O3, has a wide size distribution and irregular shapes, leading to the inhomogeneous product after the solid-state reaction.
N2 adsorption/desorption isotherms of the prepared materials are shown in Fig.1(c). All the samples display III-type isotherm curves with no hysteresis loop, indicating the materials are non-porous. The BET surface areas and average particle sizes based on this test are listed in Table 1. Na0.44MnO2 nanorods show the highest BET surface area of 4.1 m2/g and the lowest average particle size of 1.45 μm, while Na0.44MnO2 bulk have the lowest BET surface area of 0.9 m2/g and largest average particle size of 6.64 μm. The BET surface area of Na0.44MnO2 microrods is 2.7 m2/g, and its average particle size is 2.20 μm. The trend that the three materials show in the isotherms is consistent with the SEM images.
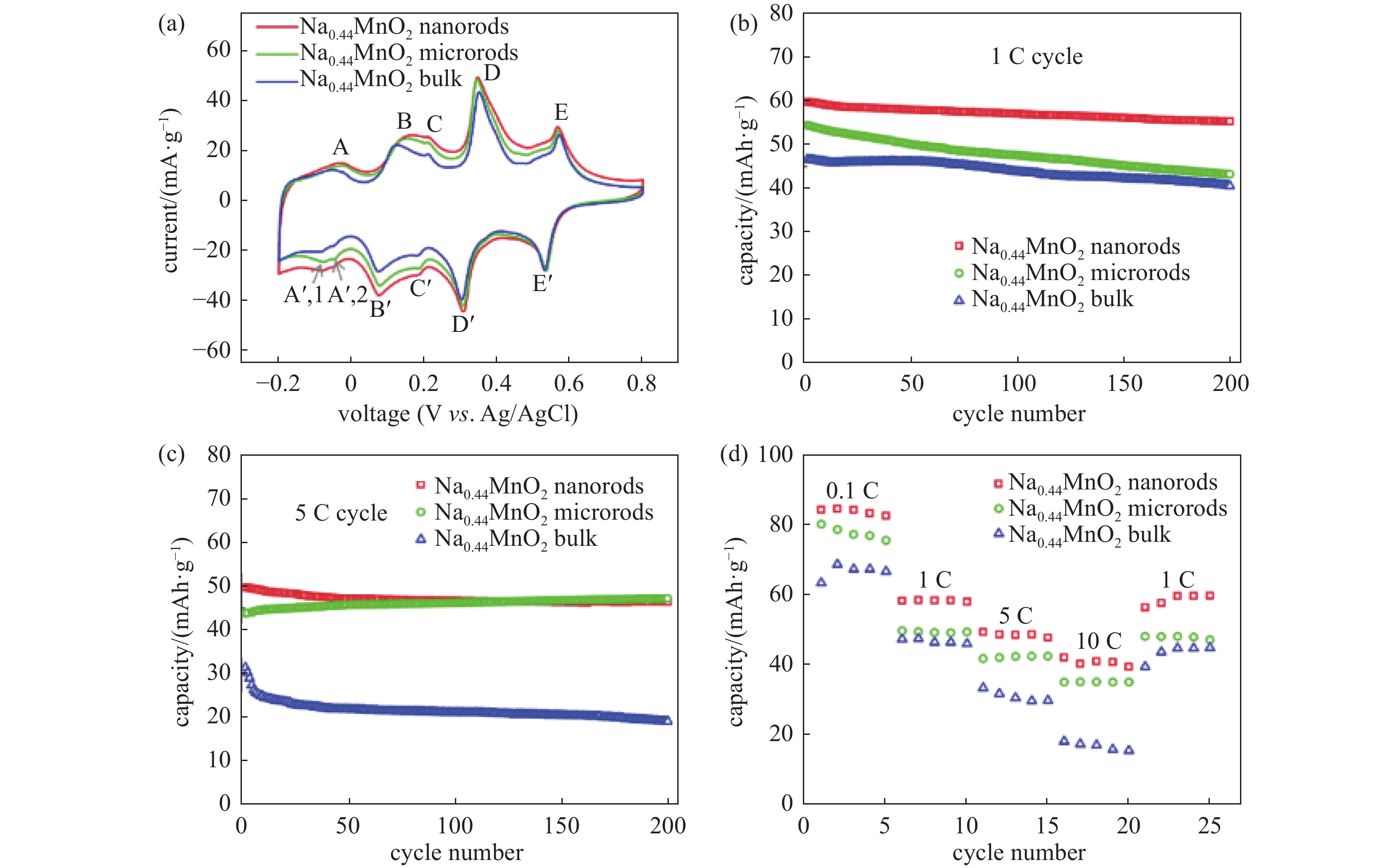
Fig. 2(a) shows the typical CV curves (6th CV cycle) of the three materials. Five anodic peaks can be observed for all the three materials, i.e., peaks A, B, C, D, and E. Peak A corresponds to the extraction of Na in the Na3 site in the S-shaped tunnel (Fig. 1(b)). [24-26] Peaks B, D, and E are related to the 3-step Na extraction in the Na2 site in the S-shaped tunnel. Peak C is due to the extraction of Na in O-shaped tunnel (Na1 site). Multiple cathodic peaks are also observed, i.e., peaks A’ (A’,1 and A’,2), B’, C’, D’, and E’, corresponding to A, B, C, D, and E, respectively. There are two cathodic peaks (A’,1 and A’,2) corresponding to the anodic peak A, indicating the insertion/extraction of Na in the Na3 site is 2-step processes. The differences between the cathodic (2 peaks) and anodic (1 peak) processes for Na insertion/extraction in the Na3 site could be due to different kinetics during Na insertion/extraction. Only one anodic peak A observed is possibly because of the overlapping of the 2-step process.
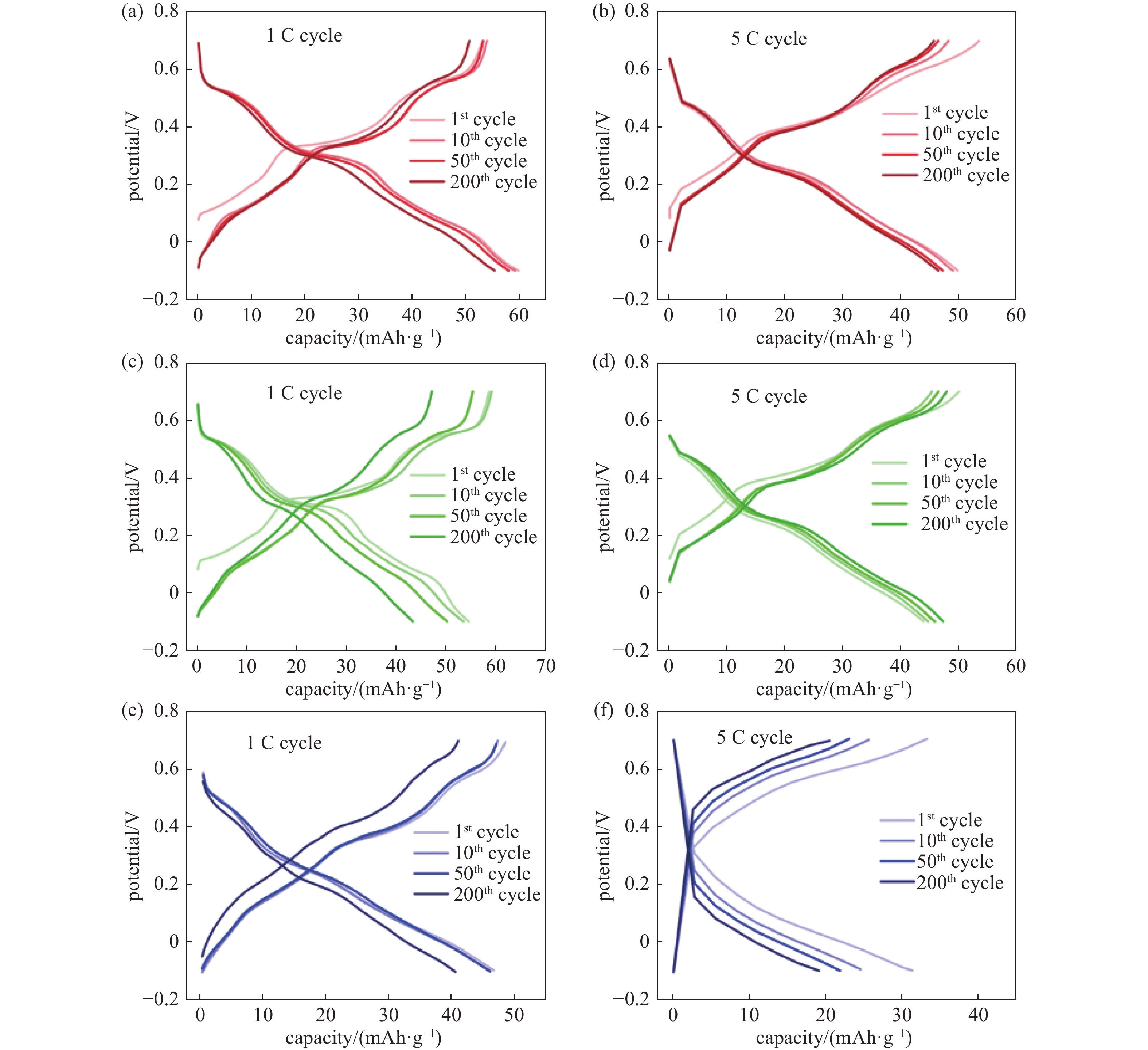
Cycle performances of the three cathodes can be observed in Fig. 2(b) and (c). The corresponding galvanostatic charge/discharge (GCD) results of the three cathodes can be seen in Fig. 3. It is observed in GCD results that Na0.44MnO2 bulk cathode shows much higher polarization than the other 2 cathodes, especially at 5 C cycle. It should be attributed to the size differences of the materials. Na0.44MnO2 bulk has the largest particle size and the smallest BET surface area which could lead to the poorest conductivity and highest polarization among the three materials. Moreover, the initial discharging capacity of Na0.44MnO2 nanorods cathode is 60 mAh/g, and 55 mAh/g is retained after 200 cycles. Na0.44MnO2 microrods cathode delivers a capacity of 55 mAh/g in the 1st cycle and maintains 44 mAh/g in the 200th cycle. In contrast, the Na0.44MnO2 bulk cathode can only deliver a 46 mAh/g capacity in the first cycle, which decreases to 40 mAh/g after 200 cycles. The Na0.44MnO2 nanorods cathode exhibits a 37.5% higher capacity than Na0.44MnO2 bulk cathode due to the size difference of the active material. The polarization of Na0.44MnO2 bulk cathode increases severely at 5 C, while the polarization of Na0.44MnO2 nanorods cathode keeps stable in the first 50 cycles. The discharging capacity of Na0.44MnO2 nanorods cathode reaches 53 mAh/g at 5 C in the first cycle and maintains 47 mAh/g after 200 cycles. Na0.44MnO2 microrods cathode delivers a 44 mAh/g in the 1st cycle, while its capacity gradually increases with cycling and attains 47 mAh/g in the 200th cycle. This cycling capacity is the same as the Na0.44MnO2 nanorods cathode in the 200th cycle. In contrast, Na0.44MnO2 bulk cathode can only deliver 32 mAh/g in the initial cycle when discharging, and this drops to 19 mAh/g after 200 cycles. The cycling capacity of Na0.44MnO2 nanorods at 5 C is 147 % higher than that of Na0.44MnO2 bulk cathode. The Na0.44MnO2 nanorods have the largest specific surface area and the smallest average particle size, which is good for the contact between the active material and the electrolyte. Hence, this cathode delivers the highest cycling capacity at 1 C and at 5 C.
The Na0.44MnO2 nanorods cathode exhibits correspondingly higher capacities in the rate test (Fig. 2(d)). The nanorod cathode delivers a very high capacity of 84 mAh/g at 0.1 C in the second cycle, 58 mAh/g at 1 C, 49 mAh/g at 5 C, 41 mAh/g at 10 C, respectively. It recovers to 56 mAh/g in the second cycle back at 1 C. All these capacities are the highest among the three cathodes. Na0.44MnO2 microrods cathode shows lower capacities than Na0.44MnO2 nanorods cathode and higher capacities than Na0.44MnO2 bulk cathode at each rate. Meanwhile, the bulk cathode can only deliver 63, 47, 33, and 18 mAh/g at 0.1, 1, 5, and 10 C, respectively. It can only recover to just 39 mAh/g when the rate is back to 1 C. All these capacities are the lowest among the cathodes. The excellent rate performance of Na0.44MnO2 nanorods cathode implies wide applications at different rates.
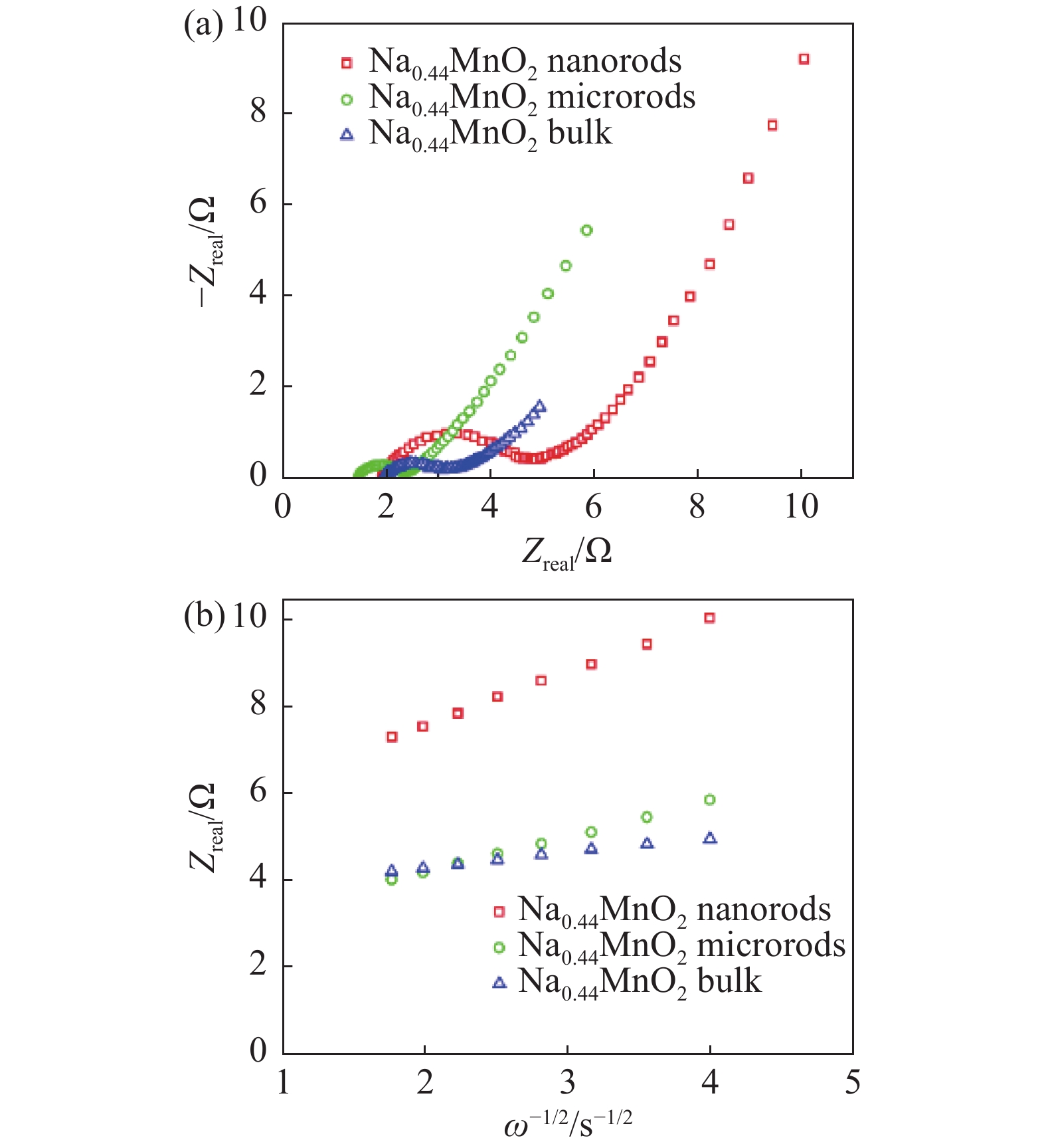
To reveal the cause of the higher cycling capacity and the higher rate capacity of Na0.44MnO2 nanorods cathode, EIS of the cathodes has been conducted. Fig. 4 shows the Nyquist plots of three pristine cathodes. All the Nyquist plots display a semicircle at medium frequency followed by a slope at low frequency. The value that the semicircle begins corresponds to Rs (ohmic resistance of the electrode and electrolyte). The diameter of the half-circle corresponds to Rct (charge transfer resistance). The slope depends on the diffusivity of ions that are conducted in the electrode. The Na0.44MnO2 bulk cathode shows a Rs and Rct values of 2.0 and 1.2 Ω, respectively. The Na0.44MnO2 nanorods cathode shows a Rs of 1.9 and a Rct and 3.0 Ω, respectively. The Na0.44MnO2 microrods cathode shows a Rs of 1.5 and a Rct and 0.9 Ω, respectively. The three cathodes have the close ohmic resistances. For Rct, The Na0.44MnO2 nanorods cathode owns the highest Rct among the three cathodes, while the Na0.44MnO2 microrods cathode owns lowest Rct.
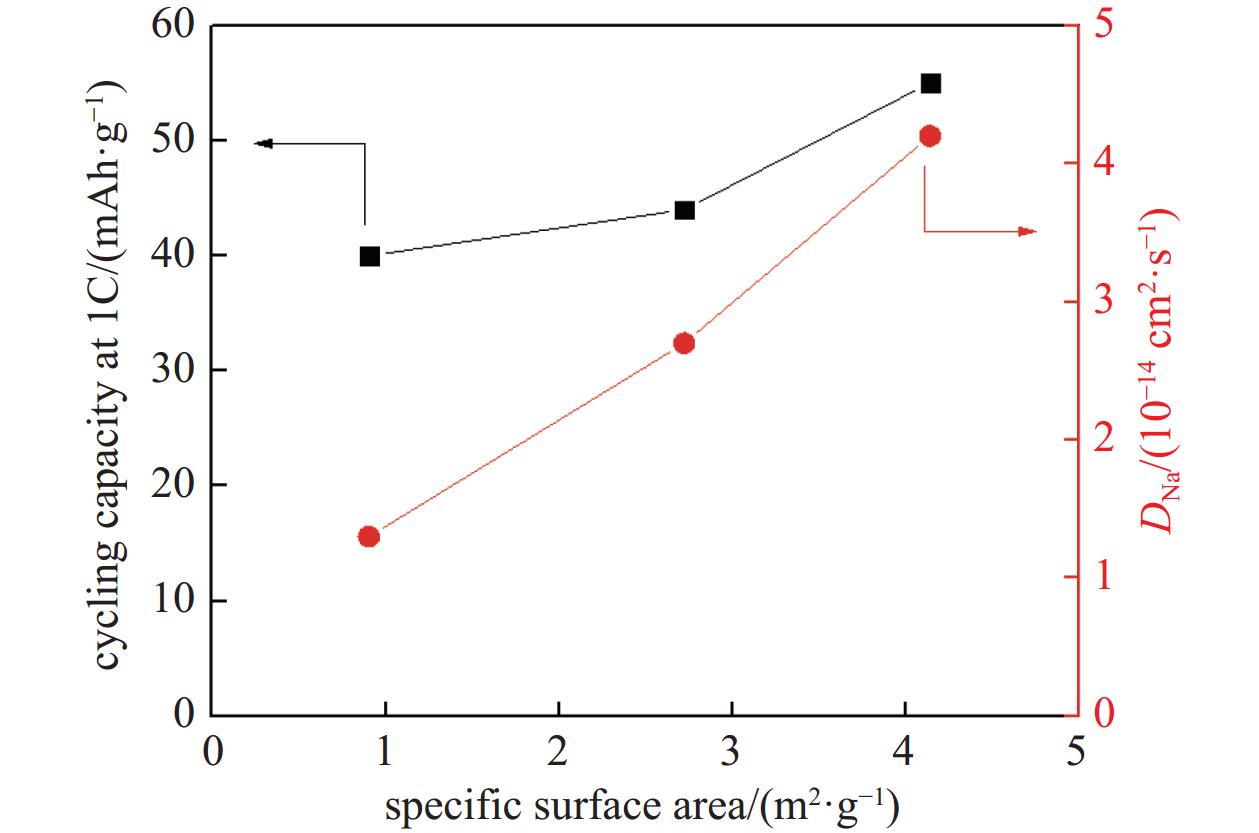
The diffusivity of Na ion (DNa) on the cathodes can be calculated to check the kinetics of the cathodes (Table 2). The equation calculating DNa is shown as following: DNa=R2T2/2A2n4F4C2σ2, where R is gas constant, T is the testing temperature, A is the surface area of the cathode, n is the number of transferred electrons, F is Faraday constant, C is the concentration of the Na ion in the electrode, σ is Warburg coefficient[26]. By plotting Zreal and ω−1/2 at low frequency, σ can be obtained by calculating the slope of the fitting line. DNa of Na0.44MnO2 nanorods cathode is calculated to be 4.2×10−14 cm2s−1. DNa of Na0.44MnO2 microrods cathode is calculated to be 2.7×10−14 cm2s−1 which is smaller than that of Na0.44MnO2 nanorods cathode. The Na0.44MnO2 bulk cathode has the smallest DNa of 1.3×10−14 cm2s−1. The largest DNa of Na0.44MnO2 nanorods cathode demonstrates that the nanorods structure, with the highest BET surface area and the smallest particle size, can facilitate the Na ion transfer in the active materials during electrochemical reactions. This high Na ion can diffusivity contributes to the high capacity of this cathode. The relationship between cycling capacity, DNa, and specific surface area is displayed in Fig. 5. It can be observed that the larger specific surface area corresponds to the higher DNa and leads to the larger cycling capacity at 1 C. The similar trends of the two profiles in this figure reveal the potential influence of DNa on the cycling capacity. It can be deduced that if the BET surface area of Na0.44MnO2 turns larger, its DNa can be higher, and the cycling capacity of the cathode can be further enhanced.
The crystal information of the electrodes before and after the cycling test can be studied by XRD and HRTEM. XRD patterns of the samples and the corresponding cathodes before and after the cycling test are illustrated in Fig. 6(a), (c), and (e). No difference is shown in peak positions between the patterns of the cathodes before and after the cycling tests, respectively. It means no new phase could be observed in the XRD patterns. The crystal lattices of the three cathodes after 5 C cycling are found in HRTEM images, Fig. 6(b), (d), and (f), respectively. The SAED patterns can confirm the existence of the crystals. The interplanar spacing of 0.28 nm and 0.45 nm can be correlated to (0 0 1) and (2 0 0) crystal plane, while the interplanar spacing of 0.26 nm can be correlated to (3 5 0) crystal plane. These parameters are the same as those of the active materials. The single crystal morphology and the maintained crystal interplanar spacings demonstrate that after 200 cycles at a high rate of 5 C, these three cathodes keep the lattices, and no phase transition happens after the cycling tests.
-
In summary, Na0.44MnO2 with different sizes have been prepared by reducing KMnO4 in hot water followed by heat treatment with NaHCO3. They are used in cathodes for aqueous Na-ion batteries. The nanorods with a width of ~ 200 nm gives the best cycling capacity and rate capacity. The initial discharging capacity of Na0.44MnO2 nanorods cathode attains 60 mAh/g at 1 C and it can remain 55 mAh/g after 200 cycles which is 37.5% higher than Na0.44MnO2 bulk cathode. At 5 C, the discharging capacity of Na0.44MnO2 nanorods cathode reaches 53 mAh/g in the first cycle and maintains 47 mAh/g after 200 cycles which is 147% higher than that of Na0.44MnO2 bulk cathode. Besides, the original orthorhombic Na0.44MnO2 phase of the Na0.44MnO2 nanorods maintains well after a high-rate cycling test at 5 C. This study demonstrates that the size of Na0.44MnO2 matters in the cathode performance. It indicates that reducing the size of active materials can be a promising way to explore high-capacity cathodes for aqueous Na-ion batteries.
Na0.44MnO2颗粒尺寸对其作为水系钠离子电池正极性能的影响
Tuning Particle Size of Na0.44MnO2 for Aqueous Na-Ion Battery Cathode
-
摘要: 水溶性钠离子电池是一种与锂离子电池相辅相成的技术,因其相对较低的成本、改善的安全性和环境友好的电解液而备受青睐。然而,较低的电极容量限制了这种电池的应用。Na0.44MnO2是一种用于钠离子电池的高容量阴极材料,其理论容量为121 mAh/g。文章研究了Na0.44MnO2的尺寸效应对阴极性能的影响。纳米棒通过热处理MnO2纳米片前驱体制备而成,其尺寸通过CTAB和KMnO4的比例进行调控。然后,Na0.44MnO2纳米棒被用作水溶性钠离子电池的活性材料。纳米棒阴极在1 C的初始循环中提供了60 mAh/g的容量,并在经过200个循环后保持了55 mAh/g,比Na0.44MnO2块状阴极高出37.5%。在高倍率的5 C下,该阴极在经过200个循环后仍能提供47 mAh/g的高容量。容量的增加归因于减小的电荷传递阻抗和纳米棒具有较高比表面积所带来的改善的钠离子扩散性能。Abstract: Aqueous sodium-ion battery is a complementary technique to lithium-ion battery because of its comparatively low cost, improved safety, and environmentally friendly electrolyte. However, the lower electrode capacity limits the application of this kind of batteries. Na0.44MnO2 is a high capacity cathode for Na-ion batteries. Its theoretical capacity is 121 mAh/g. In this article, we investigate the size effect of Na0.44MnO2 on cathode performance. The nanorods are prepared by heat treatment of a MnO2 nanoflake precursor, of which the size is tuned by the ratio of CTAB and KMnO4. The Na0.44MnO2 nanorods are then used as the active material of cathode for aqueous sodium-ion batteries. The nanorod cathode delivers 60 mAh/g in the initial cycle at 1 C and retains 55 mAh/g after 200 cycles, which is 37.5% higher than Na0.44MnO2 bulk cathode. This cathode gives a high capacity of 47 mAh/g after 200 cycles at a high rate of 5 C. The increased capacity is attributed to diminishing charge transfer resistance and improved Na-ion diffusivity caused by the higher specific surface area of the nanorod.
-
Key words:
- Na-ion battery /
- Cathode /
- Aqueous electrolyte /
- Size .
-
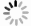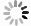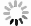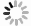
-
图 1 材料表征。(a)三个Na0.44MnO2样品的XRD粉末衍射图,(b) Na0.44MnO2晶体结构示意图,(c)三个样品的氮气吸附脱附曲线,插图为比表面积比较示意图,(d)(e)和(f)三个样品的高分辨投射电镜照片,(g)(h)和(i)三个样品的扫描电镜照片
Figure 1. Materials characterizations. (a) XRD patterns of the three samples. (b) Crystal structure of Na0.44MnO2. (c) N2 adsorption/desorption isotherms curves of the three samples, inset is the specific surface areas of the samples. HRTEM images of (d) Na0.44MnO2 nanorods (e) Na0.44MnO2 microrods, and (f) Na0.44MnO2 bulk; the scales are 5 nm. SEM images of (g) Na0.44MnO2 nanorods, (h) Na0.44MnO2 microrods, and (i) Na0.44MnO2 bulk, Mag= 10 kx, the scales are all 1 μm
图 2 电化学表征。(a)三个Na0.44MnO2样品的稳定态循环伏安曲线(第6圈),(b)和(c)三个样品在1C和5C倍率下的循环性能,(d)三个样品的倍率性能
Figure 2. Electrochemical characterizations. (a) The 6th CV curves of Na0.44MnO2 nanorods, Na0.44MnO2 microrods, and Na0.44MnO2 bulk. The profiles start to overlap since the 6th cycle. Cycling performances of Na0.44MnO2 nanorods cathode, Na0.44MnO2 microrods cathode, and Na0.44MnO2 bulk cathode (b) at 1 C, (c) at 5 C. (d) Rate performance of Na0.44MnO2 nanorods cathode, Na0.44MnO2 microrods cathode, and Na0.44MnO2 bulk cathode
图 3 充放电性能表征。(a)和(b)Na0.44MnO2纳米棒在1C和5C倍率下第1、10、50、200圈的充放电曲线,(c)和(d) Na0.44MnO2微米棒在1 C和5 C倍率下第1、10、50、200圈的充放电曲线,(a)和(b) Na0.44MnO2大颗粒在1 C和5 C倍率下第1、10、50、200圈的充放电曲线
Figure 3. Charge/discharge properties. Galvanostatic charge/discharge curves in 1st , 10th , 50th , and 200th cycles of (a) Na0.44MnO2 nanorods cathode at 1 C, (b) Na0.44MnO2 nanorods cathode at 5 C, (c) Na0.44MnO2 microrods cathode at 1 C, (d) Na0.44MnO2 microrods cathode at 5 C, (e) Na0.44MnO2 bulk cathode at 1 C, and (f) Na0.44MnO2 bulk cathode at 5 C
图 4 电化学交流阻抗研究。(a)三个样品的电化学交流阻抗谱,(b)三个样品的Zreal和ω−1/2的关系图
Figure 4. Electrochemical impedance spectroscopy (EIS) study. (a) EIS of Na0.44MnO2 nanorods cathode, Na0.44MnO2 microrods cathode, and Na0.44MnO2 bulk cathode, (b) relationship between Zreal and ω−1/2 at low frequency of Na0.44MnO2 nanorods cathode, Na0.44MnO2 microrods cathode, and Na0.44MnO2 bulk cathode
图 6 充放电后电极的表征。(a)(c)和(e)三个样品在5 C倍率下充放电200圈后的XRD粉末衍射图,(b)(d)和(f)三个样品在5 C倍率下充放电200圈后的高分辨透射电镜照片以及相应的晶体选区电子衍射图
Figure 6. Characterizations of electrodes after charge/discharge operation. XRD patterns of the cathodes before and after 200 cycles at 5 C cycling tests and the corresponding active materials, (a) Na0.44MnO2 nanorods, (c) Na0.44MnO2 microrods, and (e) Na0.44MnO2 bulk; HRTEM images of cathodes after 200 cycles at 5 C, (b) Na0.44MnO2 nanorods cathode, (d) Na0.44MnO2 microrods cathode, and (f) Na0.44MnO2 bulk cathode, inset images are SAED patterns of corresponding cathodes
Table 1. Na/Mn ratios, specific surface areas and particle sizes of the samples
Na/Mn atom ratio BET surface area /(m2/g) Average particle size /nm Na0.44MnO2 nanorods 0.442 4.1426 1448.3825 Na0.44MnO2 microrods 0.439 2.7244 2202.2848 Na0.44MnO2 bulk 0.440 0.9038 6638.9706 Table 2. Ohmic resistance, charge transfer resistance, and diffusivity of Na ion of the cathodes
Na0.44MnO2
nanorodsNa0.44MnO2
microrodsNa0.44MnO2
bulkRs / Ω 1.9 1.5 2.0 Rct / Ω 3.0 0.9 1.2 DNa / 10−14 cm2s−1 4.2 2.7 1.3 -
[1] Nitta N, Wu F, Lee J T, et al. Li-ion battery materials: present and future[J]. Materials Today,2015,18(5):252−264 [2] Etacheri V, Marom R, Elazari R, et al. Challenges in the development of advanced Li-ion batteries: a review[J]. Energy & Environmental Science,2011,4(9):3243−3262 [3] Zeng X, Li J, Singh N. Recycling of spent lithium-ion battery: a critical review[J]. Critical Reviews in Environmental ence & Technology,2014,44(10):1129−1165 [4] Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future[J]. Nature,2012,488(7411):294−303 [5] Sun Y, Guo S, Zhou H. Exploration of advanced electrode materials for rechargeable sodium-ion batteries[J]. Advanced Energy Materials,2019,9(23):1800212.1−1800212.18 [6] Bin D, Wang F, Tamirat A G, et al. Progress in aqueous rechargeable sodium-ion batteries[J]. Advanced energy materials,2018,8(17):1703008.1−1703008.31 [7] Che H, Chen S, Xie Y, et al. Electrolyte design strategies and research progress for room-temperature sodium-ion batteries[J]. Energy & Environmental ence,2017,10(5):1075−1101 [8] Suo L, Borodin O, Wang Y, et al. "Water-in-Salt" electrolyte makes aqueous sodium-ion battery safe, green, and long-lasting[J]. Advanced Energy Materials,2017,7(21):1701189.1−1701189.13 [9] Jung Y, Hong S, Kim D. Electrochemical sodium ion intercalation properties of Na2.7Ru4O9 in nonaqueous and aqueous electrolytes[J]. Journal of the Electrochemical Society,2013,160(6):A897−A900 [10] Wang Y, Mu L, Liu J, et al. A novel high capacity positive electrode material with tunnel-type structure for aqueous sodium-ion batteries[J]. Advanced Energy Materials,2015,5(22):1501005.1−1501005.8 [11] Zhang X, Hou Z, Li X, et al. Na-birnessite with high capacity and long cycle life for rechargeable aqueous sodium-ion battery cathode electrodes[J]. Journal of Materials Chemistry A,2016,4(3):856−860 [12] Zhang Y, Yuan C, Ye K, et al. An aqueous capacitor battery hybrid device based on Na-ion insertion-deinsertion in λ-MnO2 positive electrode[J]. Electrochimica Acta,2014,148:237−243 [13] Song W, Ji X, Zhu Y, et al. Aqueous sodium-ion battery using a Na3V2(PO4)3 electrode[J]. Chem Electro Chem,2014,1(5):871−876 [14] Fernandez-Ropero A J, Saurel D, Acebedo B, et al. Electrochemical characterization of NaFePO4 as positive electrode in aqueous sodium-ion batteries[J]. Journal of Power Sources,2015,291(30):40−45 [15] Zhang Y, Ye K, Cheng K, et al. Three-dimensional lamination-like P2-Na2/3Ni1/3Mn2/3O2 assembled with two-dimensional ultrathin nanosheets as the cathode material of an aqueous capacitor battery[J]. Electrochimica Acta,2014,148:195−202 [16] Qing Z, Yong L, Jun C. Advanced organic electrode materials for rechargeable sodium-ion batteries[J]. Advanced Energy Materials,2017,7(8):1601792.1−1601792.22 [17] Wu X, Sun M, Shen Y, et al. Energetic aqueous rechargeable sodium-ion battery based on Na2CuFe(CN)6 -NaTi2(PO4)3 intercalation chemistry.[J]. Chem Sus Chem,2014,7(2):407−411 [18] Chen L, Shao H, Zhou X, et al. Water-mediated cation intercalation of open-framework indium hexacyanoferrate with high voltage and fast kinetics[J]. Nature Communications,2016,7:11982.1−11982.10 [19] Li Z, Young D, Xiang K, et al. towards high power high energy aqueous sodium-ion batteries: the NaTi2(PO4)3/Na0.44MnO2 system[J]. Advanced Energy Materials,2013,3(3):290−294 [20] Wu W, Shabhag S, Chang J, et al. Relating electrolyte concentration to performance and stability for NaTi2(PO4)3/Na0.44MnO2 aqueous sodium-ion batteries[J]. Journal of the Electrochemical Society,2015,162(6):A803−A808 [21] Whitacre J F, Tevar A, Sharma S. Na4Mn9O18 as a positive electrode material for an aqueous electrolyte sodium-ion energy storage device[J]. Electrochemistry Communications,2010,12(3):463−466 [22] Wei C, Yu L, Cui C, et al. Ultrathin MnO2 nanoflakes as efficient catalysts for oxygen reduction reaction[J]. Chemical Communications,2014,50(58):7885−7888 [23] Akimoto J, Hayakawa H, Kijima N, et al. Single-crystal synthesis and structure refinement of Na0.44MnO2 . 17th International Conference on Solid Compounds of Transition Elements. Trans Tech Publications, 2011, 170: 198−202 [24] Sauvage F, Laffont L, Tarascon J M, et al. study of the insertion/deinsertion mechanism of sodium into Na0.44MnO2[J]. Inorganic Chemistry,2007,46(8):3289−3294 [25] Ma G, Zhao Y, Huang K, et al. Effects of the starting materials of Na0.44MnO2 cathode materials on their electrochemical properties for Na-ion batteries[J]. Electrochim Acta,2016,222:36−43 [26] Cao Y, Xiao L, Wang W, et al. Reversible sodium ion insertion in single crystalline manganese oxide nanowires with long cycle life[J]. Advanced Materials,2011,23(28):3155−3160 -

 首页
首页 登录
登录 注册
注册


 下载:
下载: