-
微波等离子体化学气相沉积(MPCVD)已成为一种理想的单晶金刚石合成技术[1]。特别是微波谐振腔中提供的高纯净度的沉积环境、稳定的等离子体功率密度保证生长的金刚石晶体的尺寸和质量,使MPCVD与其它CVD技术相比更有优势[2-5]。MPCVD在金刚石生长过程中的主要操作参数是气体流量、微波功率、沉积气压等,在这些参数如何影响等离子体球大小、分布和性质的潜在机制方面仍不清楚。
为了研究这些过程,需要一种非侵入性诊断,如等离子体发射光谱 (OES,Optical Emission Spectrum)。OES是一种简单的原位方法,它不会干扰到反应性等离子体,并只需在微波观察窗口外就可以探测电子激发的原子、分子的相对浓度,以及局部气体温度和等离子体放电的电子温度等等离子体特性[6]。为了提高金刚石的晶体质量,气体中通常会引入O原子,一方面可以去除与氮相关的杂质并降低硅和氢杂质水平[7],另一方面也能刻蚀掉机械损伤造成的位错缺陷,有效阻止金刚石裂纹的产生[8]。CO2是一种公认的比O2更安全的气体,近年来关于在源气体引入CO2生长高质量单晶金刚石的研究也逐渐增多[9-12],CO2分解时除了能提供刻蚀非金刚石相的原子O外,也能提供少量C源,这有助于形成金刚石相。
本文通过对CH4/H2/CO2等离子体的光谱诊断,分析了MPCVD合成单晶金刚石期间高功率密度等离子体放电(50~60 W/cm3)的发射光谱,并研究了不同CO2浓度下等离子放电特性对金刚石生长质量的影响。
-
在最大微波功率为6 kW的MPCVD系统中,在表面温度为850~880℃的CVD单晶金刚石衬底上进行沉积,衬底尺寸为4.0 × 4.0 × 0.4 mm。在放入沉积室之前,需要对衬底籽晶进行预处理,通过用王水、丙酮和无水乙醇依次洗涤衬底,保持衬底的洁净与干燥。然后放入沉积室内,在流量为300/9 mL/min(标准状态)的H2/CO2等离子体中进行1 h的蚀刻,以消除表面的缺陷,刻蚀温度为880℃。接着调整微波功率为4.2 kW,沉积气压为120 Torr直接进行生长实验。H2/CH4/CO2混合气体中的CH4(按H2体积计)浓度为6%,CO2(按H2体积计)浓度为0~5%,H2、CH4流量分别为400和24 mL/min。
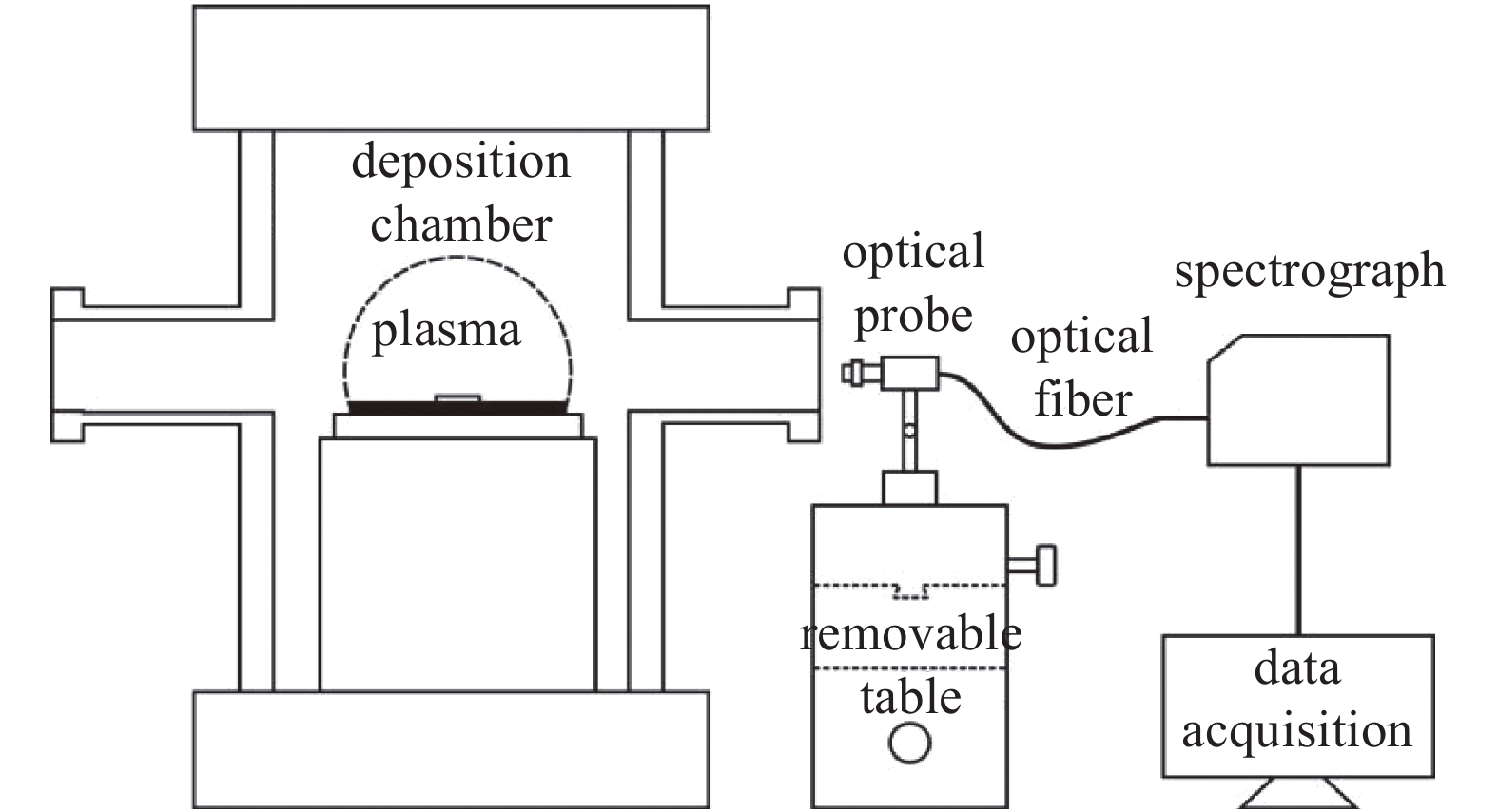
使用海洋光学Maya2000 pro高分辨率光谱仪监测沉积过程中衬底上方附近存在的等离子体种类及变化情况,通过数据线连接到光谱仪的计算机用于数据采集,设置积分时间为20 ms。光纤探头的视线穿过观察窗口并指向沉积室内的衬底正上方附近的方向。基于在衬底上方拍摄的图像中的等离子体球亮度计算等离子体体积,约为80 cm3。光纤探头固定在一个可以使其在水平(x轴)和垂直(z轴)方向上移动的带有刻度的光学平台上(如图1所示),从而实现相对于等离子体中心不同位置处的等离子体发射光谱测量。采用Raman光谱仪对单晶金刚石进行生长质量的表征。
-
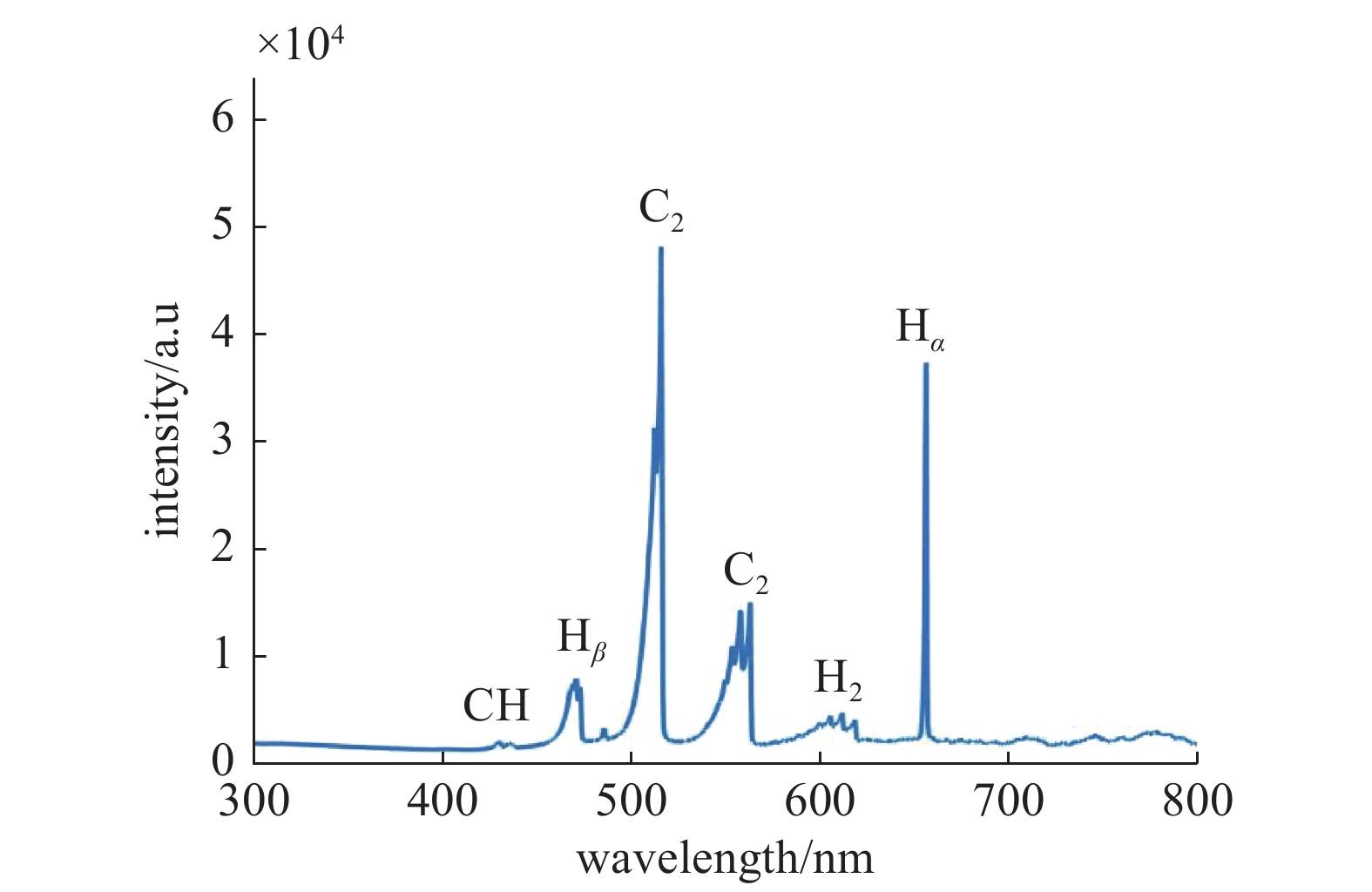
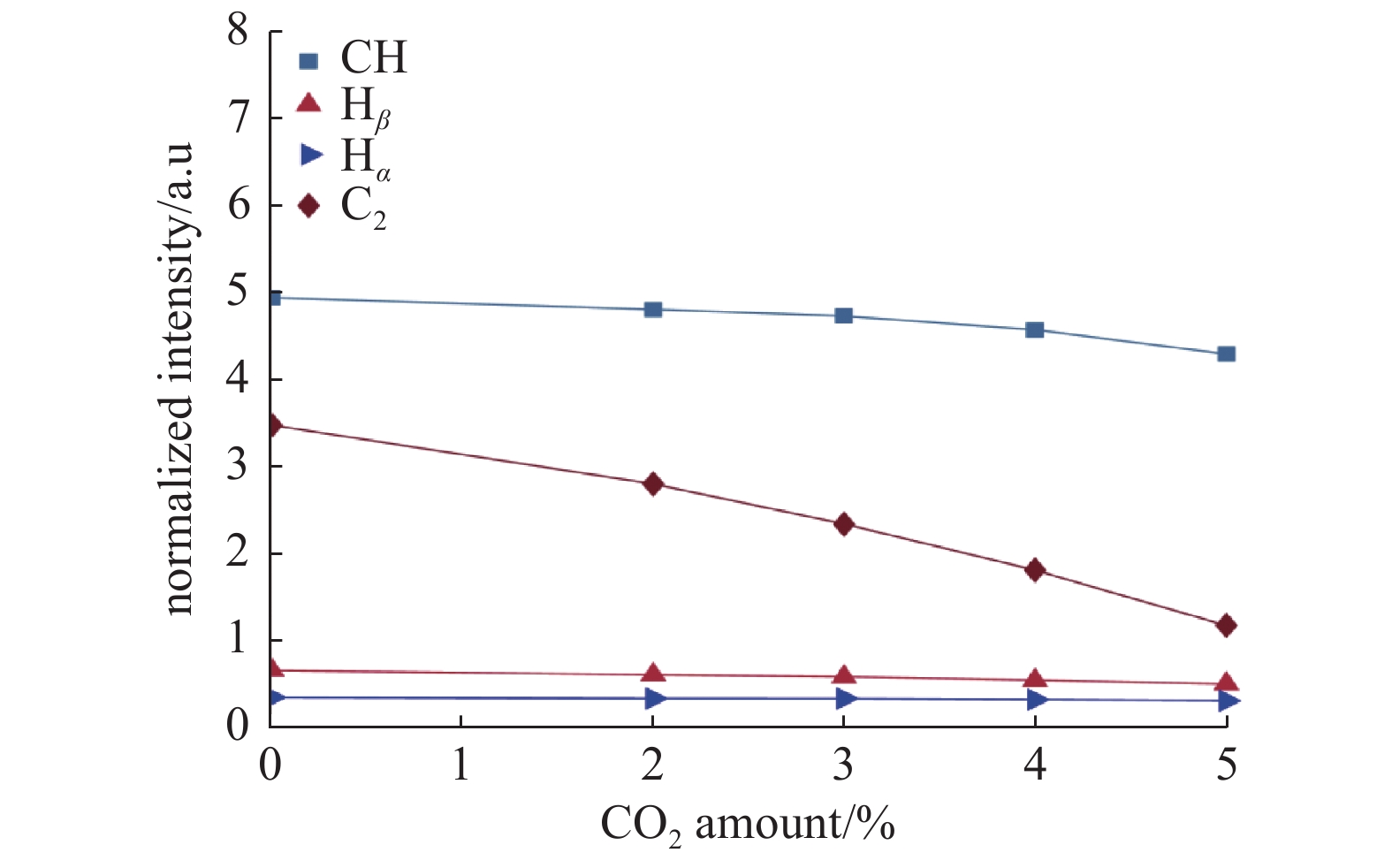
图2显示了在微波等离子体CVD沉积室内单晶金刚石生长过程中产生的等离子体的典型发射光谱。从该光谱图中得出,H2/CH4/CO2等离子体中可以观察到的主要基团是CH(430.66 nm)、C2 (516.39 nm)、C2(563.14 nm)、Hβ(486.12 nm)、H2 (602.89 nm)和Hα(656.59 nm)。图3是光谱中出现的主要基团强度随CO2浓度增加的变化关系图。可以看出,引入CO2之后,Hα和Hβ基团强度不发生明显改变,而对 CH 和 C2 基团影响较大,且都随着CO2浓度的升高而强度递减。CO2在H2/CH4等离子体中主要发生以下反应[13]:
因此,一方面将 CO2 引入等离子体中能够形成新的活性物质,例如 CO、原子氧、OH,增加了优先刻蚀石墨相的能力。在发射光谱中没有观察到含O基团,原因可能是因为OH基团更多地发生了以下反应:C2H2+OH→C2H+H2O,而处于激发态的CO在等离子体环境中很容易发生猝灭,光发射强度在几百个微秒内呈指数型衰减,激发和损失过程平衡时间在大约为1 ms[14],因此很难观察到含O基团。另一方面,高微波功率提供更多的能量,加快气体分解反应的速率,并有利于激发含C活性基团,从而促进金刚石相形成[15]。在高微波功率条件下,H2和含O基团参与的裂解反应加剧,产生更多数量的原子H和原子O,且活性增强,更容易与衬底表面充分反应,CO2的添加并没有明显提高H2和CH4的裂解反应,因而并没有产生更多的 Hα和Hβ基团,所以 Hα和Hβ基团的基团强度并没有明显变化。
-
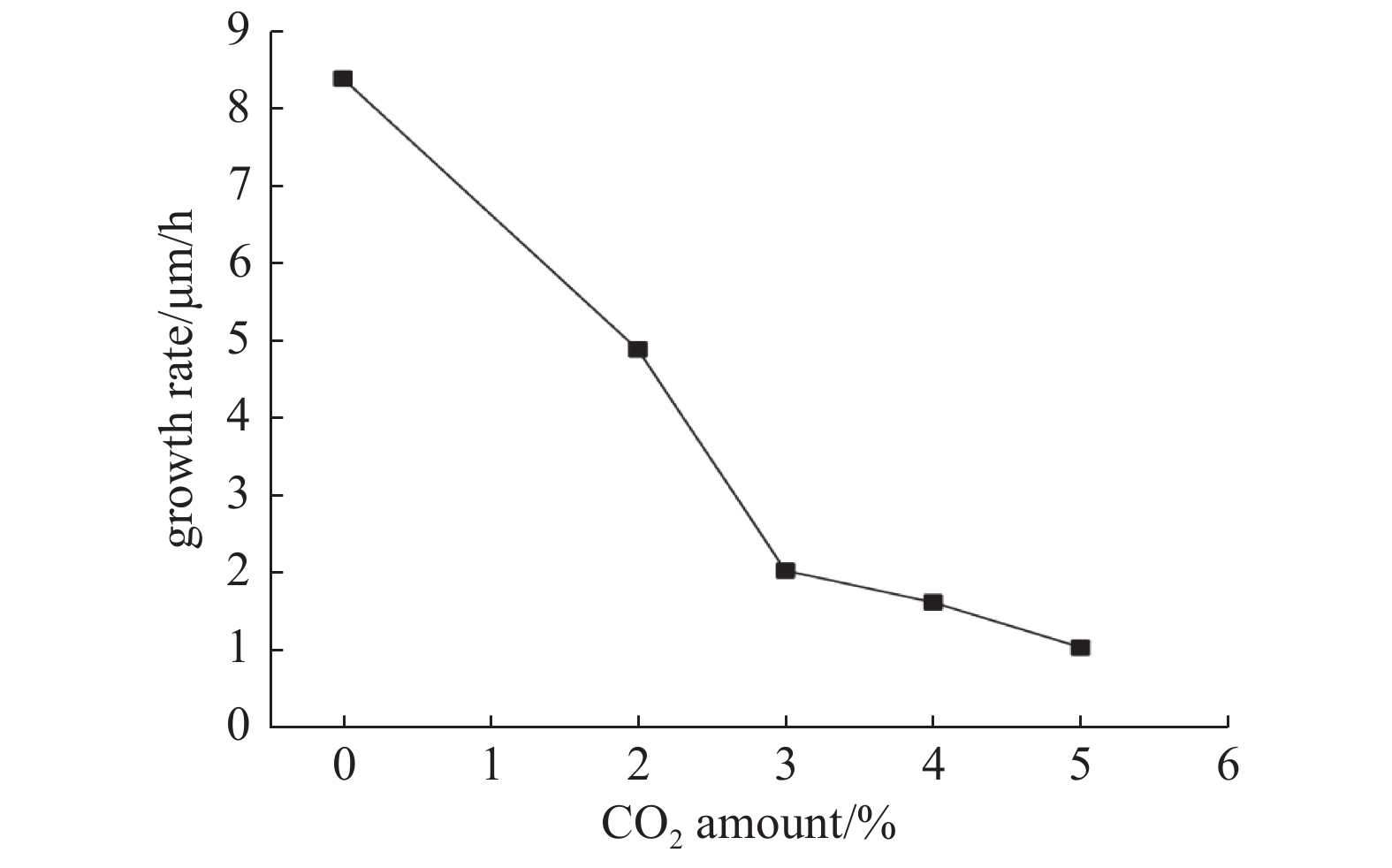
生长速率与CO2浓度变化之间的关系如图4所示。可以看出,CO2降低了金刚石生长速率,且生长速率随CO2浓度增加而逐渐降低。Mollart等[16]报告了在高气压下强烈的C2发射强度,这与金刚石生长速率的增加相关。作为单晶金刚石生长的前驱物,C2的粒子数密度很大程度上决定了金刚石单晶的沉积速率,且沉积速率通常与C2密度呈正相关关系[17]。Goyette等[18]在探究实验中发现C2 密度与C2发射强度呈线性相关关系。图3中CH和C2基团强度都随CO2浓度增加呈下降趋势,且C2下降最为明显,说明CO2会导致C2等前驱物的浓度减少,同时,原子O强烈的刻蚀能力也会导致金刚石相沉积效率变慢,从而影响了生长速率。
-
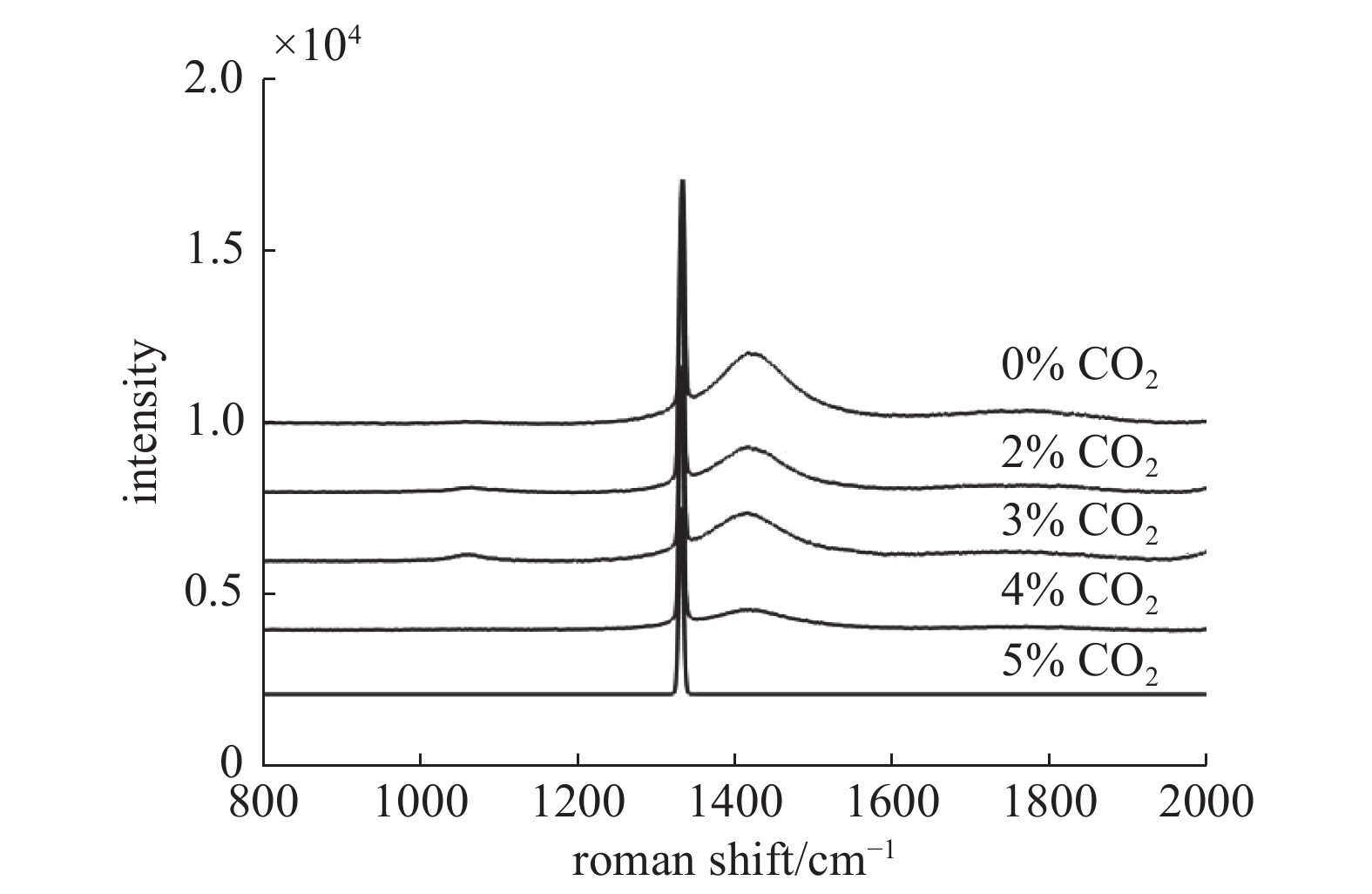
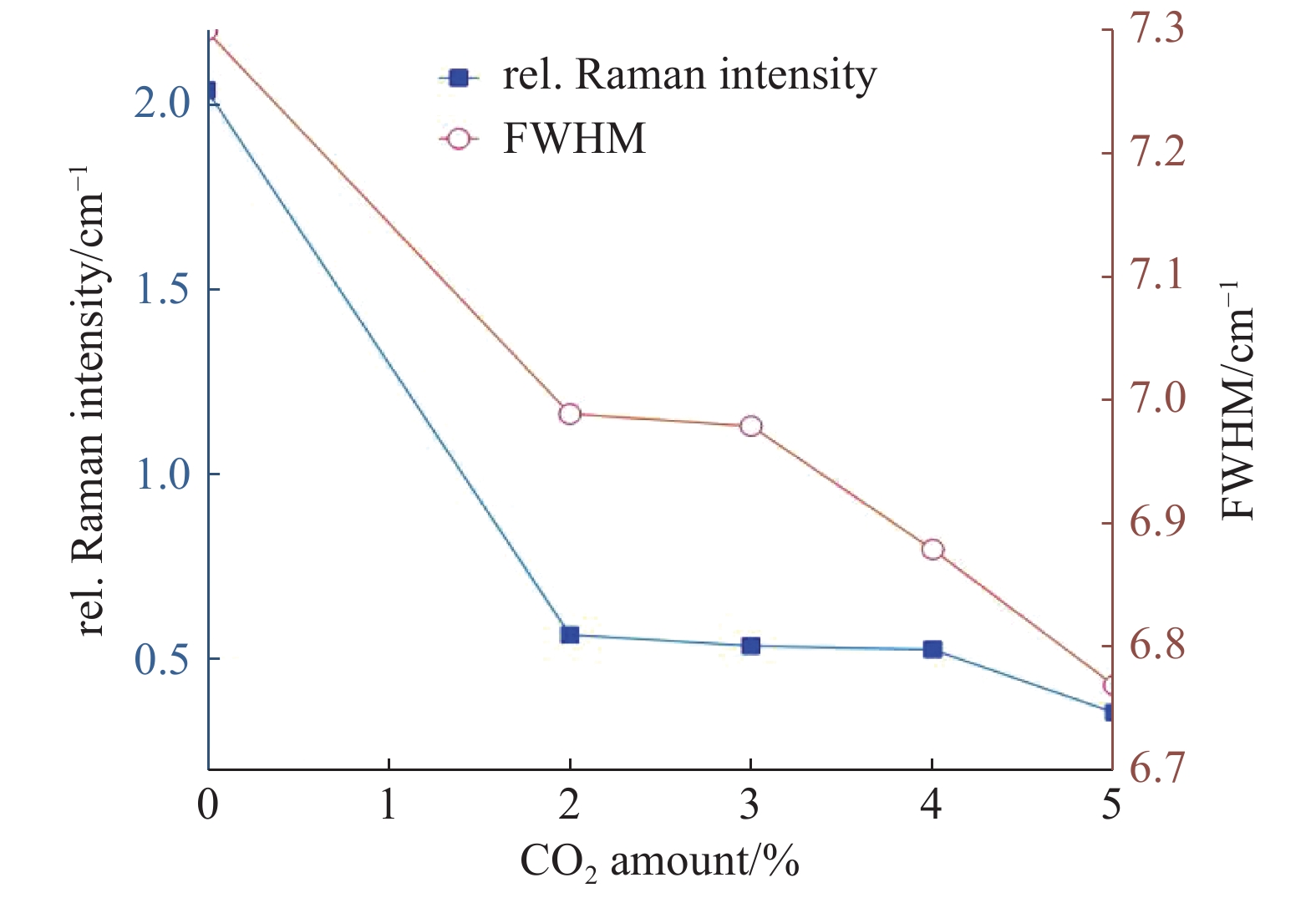
如图5所示,为0%~5%体积分数的CO2条件下生长的单晶金刚石的拉曼光谱图。从中可以看出标准本征金刚石拉曼位移1332.5 cm−1附近均出现了典型的金刚石特征峰。此外,在谱线中还出现了在1420 cm−1附近的非晶相峰,通常被认为与N-V缺陷有关[19],可能是由于腔体或腔壁中残余的微量N2参与到单晶金刚石的沉积过程中,部分杂质N取代金刚石晶格中的C原子从而形成的含N缺陷。杂质峰强度随CO2浓度增加而递减,说明N杂质含量降低。5% CO2浓度时,1420 cm−1 峰基本消失,表明对N杂质的消除能力达到了最大值。图6为单晶金刚石拉曼位移偏移量和半高宽与CO2体积分数的变化关系图,比较直观地反映出了金刚石半高宽逐渐向理想值靠近,由此可见,适量增加CO2能够降低进入金刚石晶格中的N原子数量,减少N-V缺陷的形成从而提高金刚石晶体的结晶度。金刚石特征峰的偏移量能够反映生长层的应力状态[20],添加CO2之后的金刚石拉曼位移偏移量均向高波数偏移,且有逐渐降低的趋势,证明CO2能够改善金刚石的压应力,这可能是因为CO2的增加降低了反应气体中的杂质N含量,N原子取代C原子形成C-N键会改变原来的C-C键,致使金刚石的晶格发生膨胀,从而产生更多的压应力[21]。同时,当CO2浓度增加时,分解产生了更多的原子O,在保持一定的微波功率密度条件下,进一步提高了含碳气体的活性,并产生更多的活性基团,因此生成更多的金刚石相,抑制了压应力的形成。
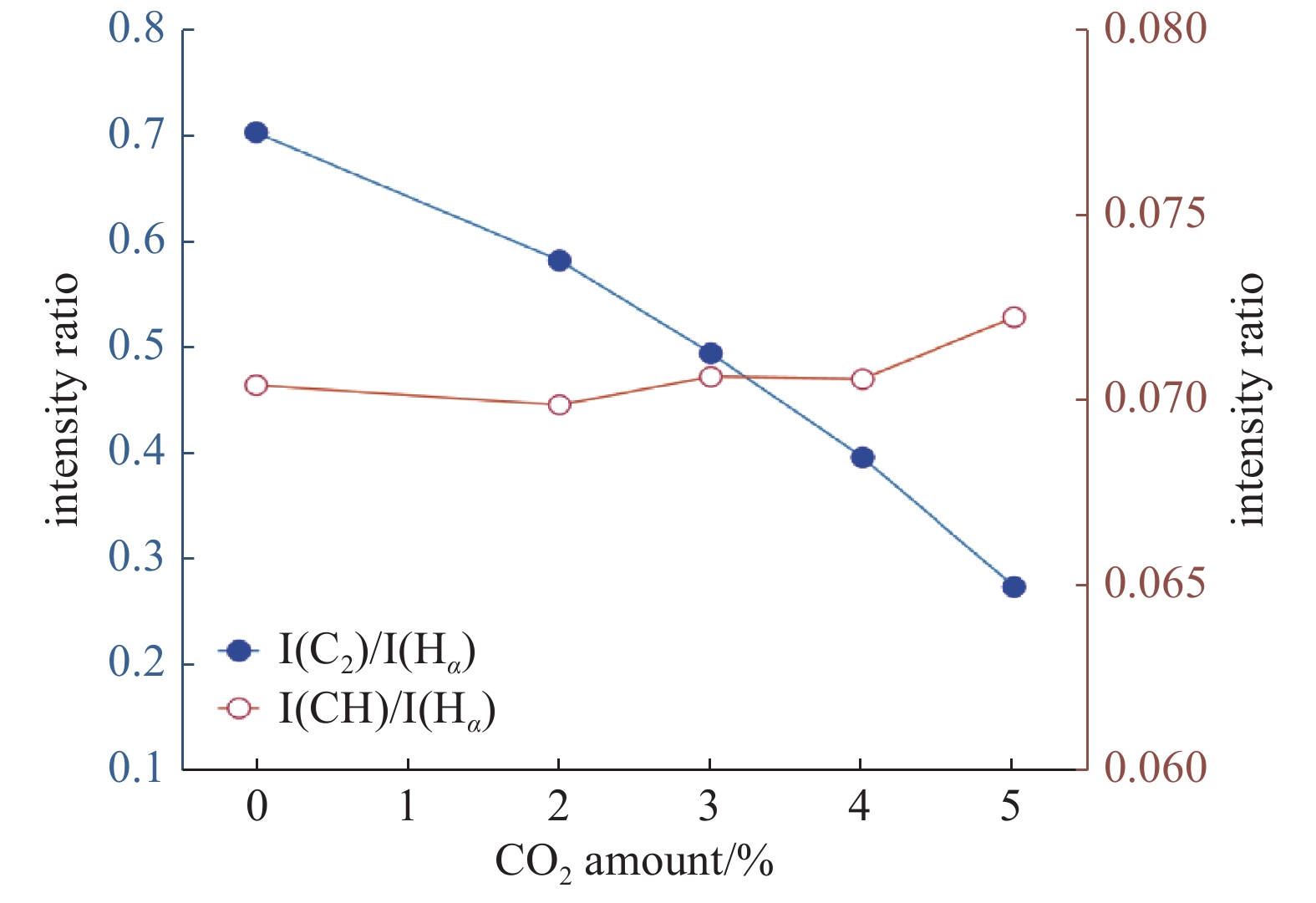
结合发射光谱图分析,等离子体内基团之间的发射光谱强度比值能够反映出彼此之间激发速率的快慢[22],并认为相对浓度比与该比值对应。图7为C2和CH基团与Hα的光谱强度比值随着CO2 体积分数增加的变化关系图。由该图可知, CO2 体积分数在 0%~5%之间变化时,I(C2)/I(Hα)值逐渐下降,而I(CH)/I(Hα)值略有上升。验证了Elliott等[23]在含CH4和CO2气体的单晶金刚石生长实验中发现的I(Hα)/I(C2)达到最大时,所沉积出的金刚石质量最好的结果。与加入O2不同的是[24],CO2浓度增加时,I(CH)/I(Hα)呈现出上升的变化趋势,是因为适当浓度的CO2产生的碳相关自由基为金刚石生长提供了碳源,导致生长金刚石的前驱物基团的相对浓度有所增加,但碍于含氧基团的刻蚀能力较强,单晶金刚石的生长速率仍是一直下降。
-
本实验探究了在添加CO2的CH4/H2等离子体中对同质外延生长单晶金刚石的影响实验,并结合等离子体发射光谱对主要基团强度随CO2体积分数变化关系进行了分析,其中CO2浓度增加对C2和CH基团强度抑制作用明显,对C2抑制作用最强。结合发射光谱图分析,C2浓度下降趋势明显且下降速率最快,可以得出CO2对金刚石前驱物的生成有不利影响的结论。拉曼光谱图中可以发现CO2对N-V缺陷有较强的刻蚀作用,并能够减少压应力的产生,因此在CO2浓度较高时能够得到高质量的单晶金刚石。从已有的拉曼光谱表征结果上看,在0%~5%CO2浓度范围内,CO2浓度为5%时对应的拉曼光谱没有观察到1420 cm−1杂质峰出现,说明该浓度下的单晶金刚石质量最好。与加入O2不同的是,在添加CO2的生长实验中,I(CH)/I(Hα)比值略有增加,说明CO2增加对非金刚石相的刻蚀能力的同时,产生的碳相关自由基也为金刚石生长提供了少量碳源,这在一定程度上减弱了含氧基团对生长速率的不利影响。
利用发射光谱探究CO2对MPCVD法生长单晶金刚石质量的影响
Exploring the Effect of CO2 on the Quality of Single Crystal Diamond Drown by MPCVD with Optical Emission Spectrum
-
摘要:
等离子体发射光谱作为一种非侵入性等离子体诊断手段能有效探测等离子体内部基团的变化信息,对这些信息的分析可以反映等离子体的特性,从而有助于探究影响单晶金刚石生长结果的原因和机理。CO2是一种比O2更安全的气体,近年来在源气体引入CO2生长高质量单晶金刚石的研究日渐增多。本文利用微波等离子体化学气相沉积法在4.2 kW的微波功率下进行单晶金刚石同质外延生长实验,对生长过程中的CH4/H2/CO2等离子体进行了发射光谱诊断,最后结合光谱信息和拉曼光谱表征研究了CO2体积分数对单晶金刚石生长质量的影响,结果发现CO2浓度增加对C2和CH基团强度抑制作用 明显,对C2抑制作用最强,这也是导致生长速率下降的主要原因。I(CH)/I(Hα)比值略有增加,说明CO2增加对金刚石前驱物的沉积有促进作用,这在一定程度上减弱了对生长速率的不利影响。拉曼表征结果说明0~5%CO2浓度下的单晶金刚石质量随CO2浓度上升变好,且浓度为5%时,1420 cm−1杂质峰基本消失。 Abstract: As a non-invasive plasma diagnostic tool, optical emission spectrum (OES) can detect a variety information of radicals in plasma, and it is possible to analyze this information to reflect the characteristics of plasma, which contribute to investigating the cause and mechanism for single crystal diamond (SCD) depositing. CO2 is a safer gas than O2, so there have been more and more studies on introducing CO2 into source gas for high-quality SCD deposition in recent years. In this paper, homoepitaxial SCDs are cultivated by microwave plasma chemical vapor deposition (MPCVD) with an input power of 4.2 kW. CO2 is added into CH4/H2 plasma, and radicals are diagnosed via OES, and then the effect of CO2 concentration on the growth quality of SCDs is investigated by combining OES information and Raman spectroscopy characterization. The results show that the increase in CO2 concentration has an obvious inhibitory effect on the intensity of C2 and CH radicals, and the strongest inhibitory effect on C2 especially, which is also the main reason for the decline in growth rate. The ratio of I(CH)/I(Hα) increased slightly, indicating that the increase of CO2 promoted the deposition of diamond precursors, which weakened the adverse effect on the growth rate to a certain extent. The Raman characterization results show that the quality of SCD grown under the concentration of 0~5% CO2 improves with the increase of CO2 concentration, and the impurity peak of 1420 cm−1 basically disappears when the concentration is 5%. -

-
-
[1] Widmann C J,Müller-Sebert W,Lang N,et al. Homoepitaxial growth of single crystalline CVD-diamond[J]. Diamond and Related Materials,2016,64:1−7 doi: 10.1016/j.diamond.2015.12.016 [2] Silva F,Hassouni K,Bonnin X,et al. Microwave engineering of plasma-assisted CVD reactors for diamond deposition[J]. Journal of physics:condensed matter,2009,21(36):364202 doi: 10.1088/0953-8984/21/36/364202 [3] Huang P,Wang J H,Liu F,et al. Research progress in preparation of mono-crystal diamond by microwave method[J]. Journal of Wuhan Institute of Technology,2016,38(4):357−363 (黄平,汪建华,刘繁,等. 微波法制备单晶金刚石的研究进展[J]. 武汉工程大学学报,2016,38(4):357−363(in chinese) doi: 10.3969/j.issn.1674-2869.2016.04.009 Huang P, Wang J H, Liu F, et al. Research Progress in Preparation of Mono-Crystal Diamond by Microwave Method[J]. Journal of Wuhan Institute of Technology, 2016, 38(04): 357-363 doi: 10.3969/j.issn.1674-2869.2016.04.009 [4] Vikharev A L,Lobaev M A,Gorbachev A M,et al. Investigation of homoepitaxial growth by microwave plasma CVD providing high growth rate and high quality of diamond simultaneously[J]. Materials Today Communications,2020,22:100816 doi: 10.1016/j.mtcomm.2019.100816 [5] Gao D,Ma Z B. Two-dimensional expansion of single crystal diamond growth by MPCVD[J]. Journal of Vacuum Science & Technology,2022,42(7):541−546 (高登,马志斌. MPCVD二维扩大生长单晶金刚石[J]. 真空科学与技术学报,2022,42(7):541−546(in chinese) Gao D, Ma Z B. Two-Dimensional Expansion of Single Crystal Diamond Growth by MPCVD[J]. Journal of Vacuum Science & Technology, 2022, 42(07): 541-546 [6] Gicquel A,Hassouni K,Farhat S,et al. Spectroscopic analysis and chemical kinetics modeling of a diamond deposition plasma reactor[J]. Diamond and Related Materials,1994,3(4-6):581−586 doi: 10.1016/0925-9635(94)90229-1 [7] Das D,Singh R N,Barney I T,et al. Effect of oxygen on growth and properties of diamond thin film deposited at low surface temperature[J]. Journal of Vacuum Science & Technology A:Vacuum, Surfaces, and Films,2008,26(6):1487−1496 [8] Tallaire A,Achard J,Silva F,et al. Oxygen plasma pre‐treatments for high quality homoepitaxial CVD diamond deposition[J]. physica status solidi (a),2004,201(11):2419−2424 doi: 10.1002/pssa.200405164 [9] Zhang Q,Li H D,Cheng S H,et al. The effect of CO2 on the high-rate homoepitaxial growth of CVD single crystal diamonds[J]. Diamond and related materials,2011,20(4):496−500 doi: 10.1016/j.diamond.2011.02.001 [10] Richley J C,Kelly M W,Ashfold M N R,et al. Optical emission from microwave activated C/H/O gas mixtures for diamond chemical vapor deposition[J]. The Journal of Physical Chemistry A,2012,116(38):9447−9458 doi: 10.1021/jp306191y [11] Liu C,Wang J H,Xiong L W. Effect of CO2 on MPCVD preparation of diamond film[J]. Vacuum and Cryogenics,2014,20(4):234−238 (刘聪,汪建华,熊礼威. CO2对MPCVD制备金刚石膜的影响研究[J]. 真空与低温,2014,20(4):234−238(in chinese) doi: 10.3969/j.issn.1006-7086.2014.04.14 Liu C, Wang J H, Xiong L W. Effect of CO2 on MPCVD preparation of diamond film[J]. Vacuum and Cryogenics, 2014, 20(04): 234-238. doi: 10.3969/j.issn.1006-7086.2014.04.14 [12] Sun Q,Wang J H,Chen Y,et al. The effect of concentration of CO2 on the structure of diamond films[J]. Vacuum and Cryogenics,2017,23(1):58−62 (孙祁,汪建华,陈义,等. CO2对金刚石膜结构的影响[J]. 真空与低温,2017,23(1):58−62(in chinese) doi: 10.3969/j.issn.1006-7086.2017.01.011 Sun Q, Wang J H, Chen Y, et al. The Effect of Concentration of CO2 on the Structure of Diamond Films[J]. Vacuum and Cryogenics, 2017, 23(01): 58-62. doi: 10.3969/j.issn.1006-7086.2017.01.011 [13] Inspektor A,Liou Y,McKenna T,et al. Plasma CVD diamond deposition in CHO systems[J]. Surface and Coatings Technology,1989,39:211−221 [14] Bougdira J,Remy M,Alnot P,et al. Combined effect of nitrogen and pulsed microwave plasma on diamond growth using CH4–CO2 gas mixture[J]. Thin Solid Films,1998,325(1-2):7−13 doi: 10.1016/S0040-6090(97)00955-3 [15] Achard J,Silva F,Tallaire A,et al. High quality MPACVD diamond single crystal growth: high microwave power density regime[J]. Journal of Physics D:Applied Physics,2007,40(20):6175 doi: 10.1088/0022-3727/40/20/S04 [16] Mollart T P,Lewis K L. Optical-quality diamond growth from CO2-containing gas chemistries[J]. Diamond and related materials,1999,8(2-5):236−241 doi: 10.1016/S0925-9635(98)00268-4 [17] Weng J,Zhou C,Liu F,et al. Influence of the gas flow ratio between CH4 and H2 on the growth of diamond films at high microwave power[J]. Surface Technology,2018,47(11):202−209 (翁俊,周程,刘繁,等. 甲烷与氢气的流量比在高功率下对金刚石膜生长的影响[J]. 表面技术,2018,47(11):202−209(in chinese) doi: 10.16490/j.cnki.issn.1001-3660.2018.11.029 Weng J, Zhou C, Liu F, et al. Influence of the Gas Flow Ratio between CH4 and H2 on the Growth of Diamond Films at High Microwave Power[J]. Surface Technology, 2018, 47(11): 202-209. doi: 10.16490/j.cnki.issn.1001-3660.2018.11.029 [18] Goyette A N,Lawler J E,Anderson L W,et al. Swan band emission intensity as a function of density[J]. Plasma Sources Science and Technology,1998,7(2):149 doi: 10.1088/0963-0252/7/2/009 [19] Zhu R H,Liu J L,Chen X L,et al. Research on 1420 cm−1 characteristic peak of free-standing diamond films in raman spectrum[J]. Journal of Synthetic Crystals,2015,44(4):867−871+884 (朱瑞华,刘金龙,陈良贤,等. 金刚石自支撑膜拉曼光谱1420 cm−1特征峰研究[J]. 人工晶体学报,2015,44(4):867−871+884(in chinese) doi: 10.3969/j.issn.1000-985X.2015.04.003 Zhu R H, Liu J L, Chen X L, et al. Research on 1420 cm-1 Characteristic Peak of Free-standing Diamond Films in Raman Spectrum[J]. Journal of Synthetic Crystals, 2015, 44(04): 867-871+884. doi: 10.3969/j.issn.1000-985X.2015.04.003 [20] Pal K S,Mallik A K,Dandapat N,et al. Microscopic properties of MPCVD diamond coatings studied by micro-Raman and micro-photoluminescence spectroscopy[J]. Bulletin of Materials Science,2015,38(2):537−549 doi: 10.1007/s12034-015-0860-9 [21] Ye Y Q,Kuang T C,Lei S M,et al. Technique progress in raman spectroscopy characterization of diamond or diamond film[J]. Diamond & Abrasives Engineering,2007(5):17−21 (叶永权,匡同春,雷淑梅,等. 金刚石(膜)的拉曼光谱表征技术进展[J]. 金刚石与磨料磨具工程,2007(5):17−21(in chinese) doi: 10.3969/j.issn.1006-852X.2007.05.005 Ye Y Q, Kuang T C, Lei S M, et al. Technique progress in raman spectroscopy characterization of diamond or diamond film[J]. Diamond & Abrasives Engineering, 2007(05): 17-21. doi: 10.3969/j.issn.1006-852X.2007.05.005 [22] Li C H,Liao Y,Chang C,et al. Optical emission spectroscopy for hot filament diamond growth process with nitrogen addition[J]. Journal of Inorganic Materials,2001,16(1):6 (李灿华,廖源,常超,等. 氮气氛下金刚石薄膜生长过程中的光发射谱研究[J]. 无机材料学报,2001,16(1):6(in chinese) doi: 10.3321/j.issn:1000-324X.2001.01.013 Li C H, Liao Y, Chang C, et al. Optical Emission Spectroscopy for Hot Filament Diamond Growth Process with Nitrogen Addition[J]. Journal of Inorganic Materials, 2001, 16(1): 6. doi: 10.3321/j.issn:1000-324X.2001.01.013 [23] Elliott M A,May P W,Petherbridge J,et al. Optical emission spectroscopic studies of microwave enhanced diamond CVD using CH4/CO2 plasmas[J]. Diamond and Related Materials,2000,9(3-6):311−316 doi: 10.1016/S0925-9635(99)00196-X [24] Cao W,Ma Z B. Optical spectroscopy for high-presure microwave plasma chemical vapor deposition of diamond films[J]. Spectroscopy and Spectral Analysis,2015,35(11):3007−3011 (曹为,马志斌. 高气压MPCVD沉积金刚石的光谱研究[J]. 光谱学与光谱分析,2015,35(11):3007−3011(in chinese) Cao W, Ma Z B. Optical Spectroscopy for High-Presure Microwave Plasma Chemical Vapor Deposition of Diamond Films[J]. Spectroscopy and Spectral Analysis, 2015, 35(11): 3007-3011. -


 首页
首页 登录
登录 注册
注册



 下载:
下载: